Features Brief
Veeva SafetyDocs Features Brief
Centrally manage pharmacovigilance content and collaborate globally
Veeva SafetyDocs centrally manages pharmacovigilance processes and content for greater operational efficiency and compliance. With controlled access to safety content, it also enables global collaboration within teams and across clinical, quality, regulatory, or external partners.
Business Benefits
-
Improve inspection readiness.
Detailed audit trail, version control, intuitive search, and real-time status visibility improve compliance and audit and inspection readiness. -
Enable global collaboration.
Easily share content internally or externally with partners and collaborate across quality, regulatory, clinical, and safety teams. -
Increase efficiency.
Automate processes, streamline document management, and easily centralize content.
Capabilities
-
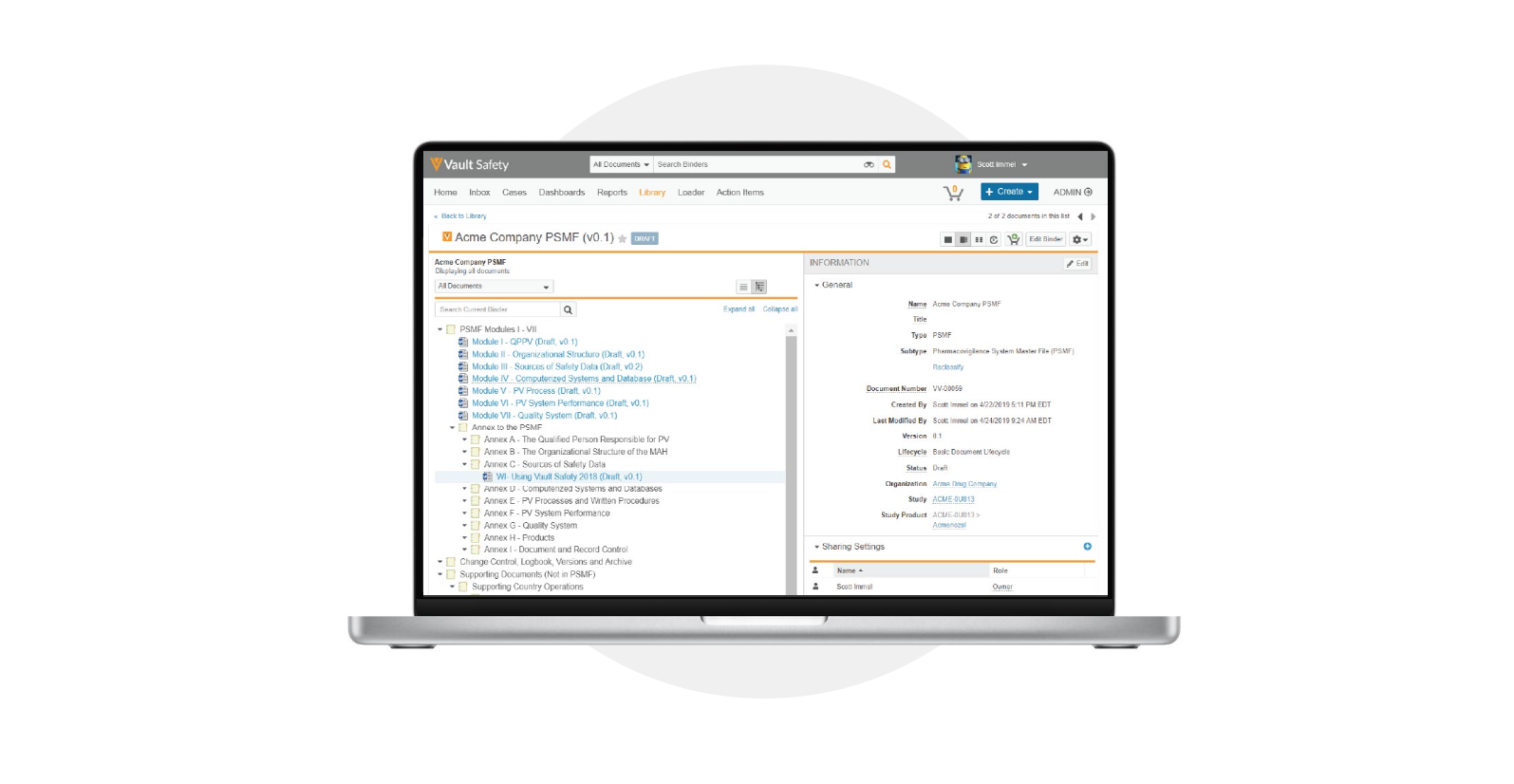
PSMF Management
Rapidly generate a complete PSMF with one-click PDF merge, including a table of contents, logbook of changes, and electronic signatures that are compliant with Title 21 CFR Part 11 and Annex 11. Quickly organize and reuse content across multiple regions to simplify global/ local PSMF submissions. -
PVA Management
Seamlessly oversee the status of pharmacovigilance agreements (PVAs) and partner and/or service provider data obligations. Link activities to the underlying commitments for easy reconciliation. -
Risk Management
Author, manage, and track core and local risk management plans (RMP) and additional risk minimization measures (aRMMs). Easily identify current plans with version management and track the implementation of aRMMs for improved oversight compliance monitoring. -
Aggregate Report Authoring
Easily plan global aggregate report submissions and track progress towards them. Create custom data extracts and automatically run data sets or generate customized line listings and data reports. Initiate a report workflow to manage tasks and actions to support the report authoring, review and approval activities. -
Signal Management
Maintain compliant GVP Module IX signal management processes and workflows that are fully integrated with MedDRA.
Features
-
Real-time Collaboration and Controlled Access
Seamless integration between Vault and Microsoft Office Online provides simultaneous authoring for real-time collaboration. Securely enable internal and external users with role-based permissions. -
Comprehensive Audit Trail
Demonstrate compliance with detailed audit trails capturing every event in a document’s history – including document approvers and reviewers, status changes, execution of a signature, and more. -
Interactive Dashboards and Reports
Self-service reporting and dashboards enable users to see the status of content and processes, including periodic reviews, making it easy to identify bottlenecks or compliance risks. Click through the report for more detail or easily share information with your team or partners. -
Configurable Workflows
Automatically route content for review and approval to align with business processes, and trigger workflows based on document expiration and periodic review notification. -
Version Control
Automate versioning and easily compare documents to previous versions to see how the content has changed. -
Electronic Signature and Manifestation
Approve documents using electronic signatures and manifestations that are compliant with Title 21 CFR Part 11 and Annex 11.