Features Brief
Veeva Study Startup Features Brief
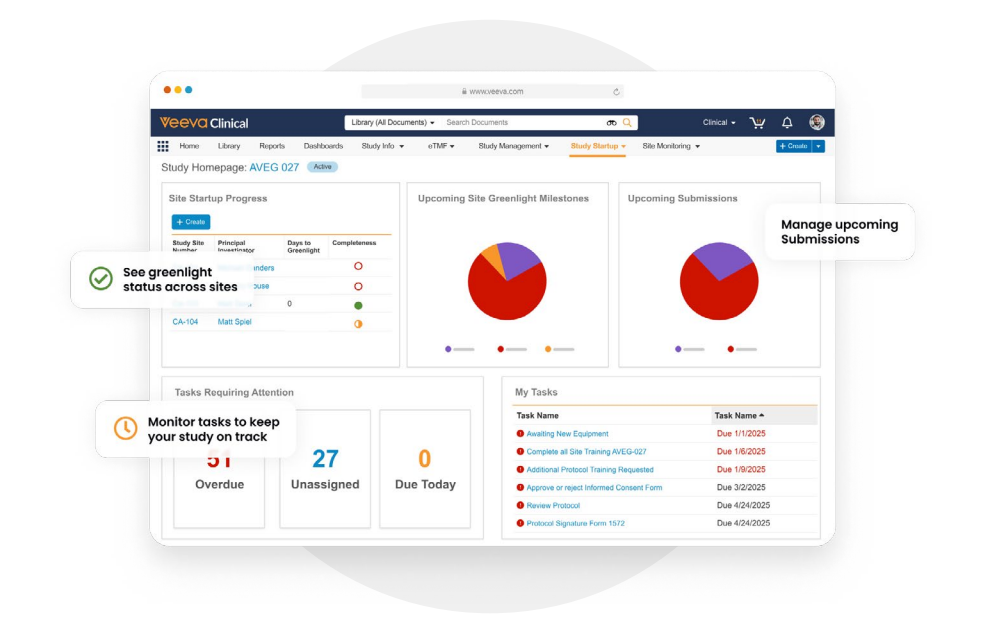
End-to-End Study Start-Up in One Application
Veeva Study Startup accelerates time to site activation by connecting global teams and enabling best practices for managing country and site start-up processes.
Because study start-up documentation and data (including milestone information, baseline, projected, and actual dates) are managed in a single system, sponsors and CROs have early visibility to bottlenecks associated with events on the critical path to activation. As a result, resources can be more efficiently allocated and adjusted based on start-up progress.
Business Benefits
-
Speed study start-up.
Streamline end-to-end study start-up processes - from site identification to site greenlight - to accelerate time to site activation. -
Make better, more informed decisions.
Actively monitor and track site activation progress with realtime dashboards and reports. -
Enable effective collaboration.
Achieve greater alignment across study partners through seamless exchange of start-up content and information.
Features
-
Site Feasibility and Selection
Easily collect investigator and facility data to make better decisions about sites that should be qualified. Distribute feasibility surveys automatically to qualifying sites for easy completion and tracking. Site selection workflows automatically trigger downstream activation activities for seamless start-up management and visibility. -
Contract and Budget Lifecycles
Streamline the contracting process by leveraging a dedicated Contracts and Budgets lifecycle. Calculate cycle times to monitor the timeliness of negotiations, assisting with better planning and faster negotiations in future studies. -
Standard Questions and Reusable Responses
Make survey completion easier for sites by shortening surveys and saving and pre-populating responses with Veeva-supported industry-standard questions. -
Submissions Tracking
Simplify submissions and track key dates, statuses, and documents needed for Health Authority submissions and Ethics Committee submissions, including EU CTR and RFI tracking. Take advantage of a productized connector with Veeva RIM for alignment between Regulatory and Clinical teams. -
Study Startup Homepage
Prioritize and manage critical tasks and milestones, including high-priority, overdue, and at-risk activities, across multiple studies through a visually intuitive homepage. Start-up specialists and managers get a quick snapshot of start-up progress, can drill down into underlying details, and take immediate action directly from the homepage. -
Global Milestones and Standard Metrics
Measure cycle times and get visibility into key performance indicators to identify areas for improvement and optimize startup processes. -
Global Workflows and Country Intelligence
Veeva Study Startup specialists gain full visibility into progress across countries, tracking sites, and required documents to quickly identify blockers to a site’s greenlight. Submission workflows ensure completeness before regulatory handoff. With 45+ built-in country workflows, teams can efficiently navigate complex country startup requirements. -
Unified Clinical Operations Platform
Veeva Study Startup shares documentation and data with other core Clinical products without complex integrations. Milestones and tasks are populated in Veeva CTMS. Start-up teams can automatically exchange essential documents with sites via Veeva Site Connect based on country-specific tailored lists, and start-up content is auto-filled in Veeva eTMF. -
Study Startup Milestones
Activities and documents required for site activation are linked to study milestones, defining the critical path to open a country and get every site to the first subject and first visit faster.
About Veeva Clinical Operations
Veeva Clinical Operations empowers clinical teams with a unified platform for efficient trial execution. Streamlined processes and improved data visibility from startup through closeout accelerates timelines and enhances collaboration across sponsors, sites, and CROs.