White Paper
Why Biopharmas are Reevaluating Safety Solutions
Simplify and automate safety operations with one unified and global solution
Biopharma companies are leveraging innovative technologies to provide cost and process efficiencies and reduce risk. With simplified and automated safety operations, pharmacovigilance teams can more easily scale with growing data volumes, meet changing regulatory / business demand, and expand into new markets.
The following are 5 key areas driving companies to reevaluate safety solutions. The guide helps you understand what is possible with current technologies and assists in your evaluation to get the best solution to meet today’s business needs. Greater Automation with a Unified and Connected Safety Solution
Global ICSR reporting
Traditional ‘hub and spoke’ model for affiliate reporting and data management is inefficient with lack of data standardization, affiliate-specific workflows, and multilingual intake and submission. Resources are also needed for periodic reconciliation of partner cases to ensure high-quality data.
Giving partners access to a global safety solution that supports multilingual and regional regulatory requirements eliminates multiple reporting systems and provides a single source of safety data for compliance tracking and real-time analysis. Modern solutions have easily configurable workflows and security and enable companies to manage - with partners - the entire individual case safety report (ICSR) reporting lifecycle. With real-time safety data for all parties and full visibility and traceability from collection to case submission, there is more timely benefit-risk evaluation, better decision-making, and improved compliance.
Touchless case processing
Supporting the complete safety case lifecycle in one unified solution also allows greater automation. From assisting case processors in key steps, such as triaging and coding, to automating the entire intake to submissions workflow with touchless case processing.Leading biopharmas are driving greater operational productivity while minimizing risk by employing rules-based automation in touchless case processing and applying it to non-serious cases of well understood products. Companies can take a risk-based approach to automation and there will more automation use cases as capabilities, understanding, and confidence increases.
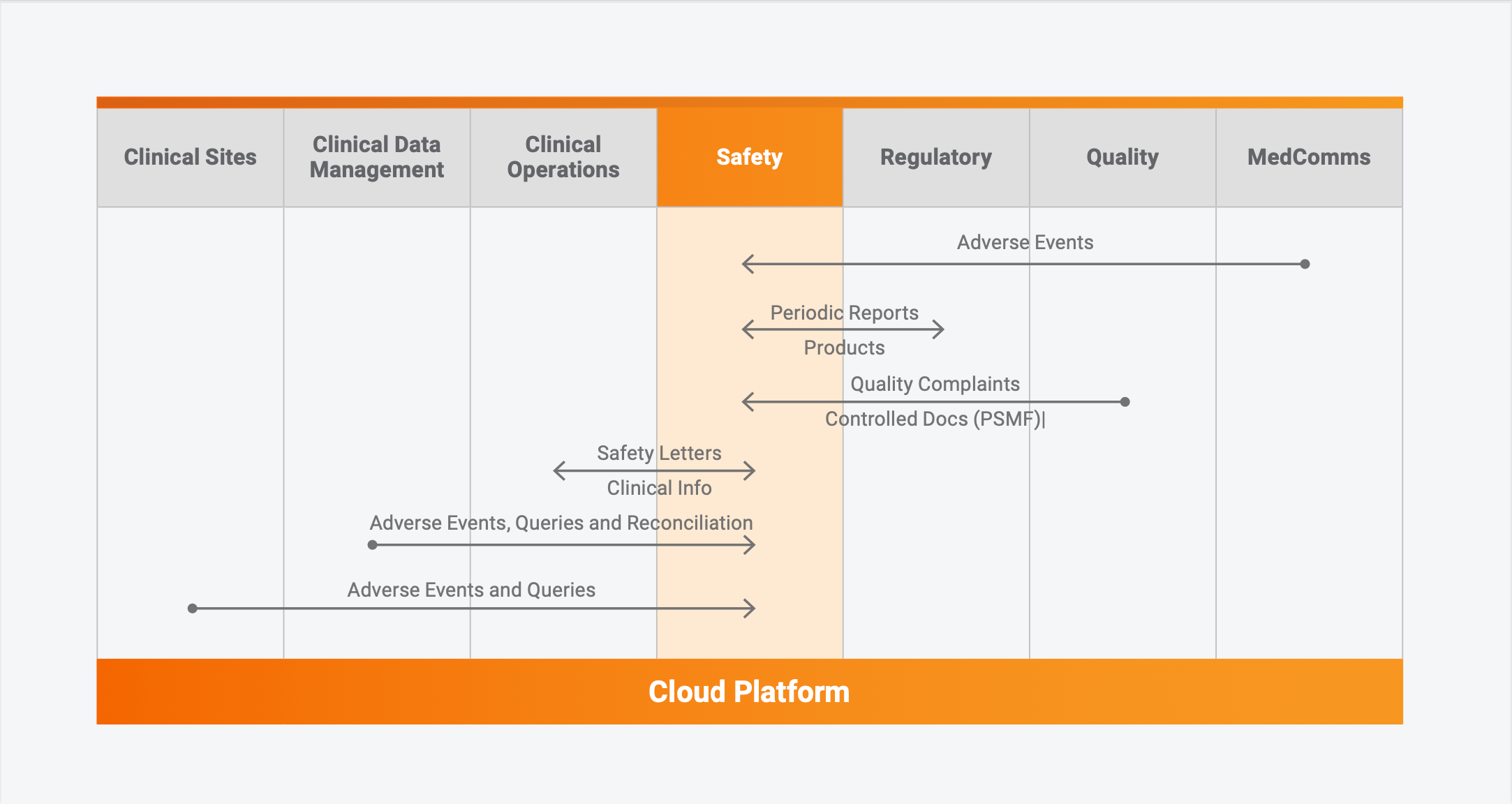
Unified safety solution for seamless aggregate report, signal, and content management Modern safety applications are built on a single cloud platform and operate together as a unified solution. Case intake, case processing, medical coding, ICSR submissions, aggregate reports, safety content management, operational reporting, signal and data analysis, and other capabilities work in harmony to streamline processes and provide a single source of safety data and content, including reference materials and documents. For example, line listing data in the ICSR application automatically populates periodic reports that are authored and managed in the safety content management system, or real-time ICSR data is accessible to safety analysis tools for earlier signal detection and faster response to queries from health authorities.
Connecting data across functional areas to eliminate duplicate data entry and reconciliation
Pharmacovigilance processes can span across functional areas and involve moving data, duplicate data entry, or data reconciliation. Reentering into a safety system clinical adverse data from an EDC system or product complaints from a quality system is manual and prone to error. Companies that develop custom integrations between systems pay a high cost to continually maintain and validate them.
With applications built on the same cloud platform, companies can more easily integrate and streamline end-to-end processes and provide a single source of pharmacovigilance data and content across the company. Providing a consistent user experience across each area and centralizing security administration, also simplifies application management and reduces end-user training. Seamless, cross-functional workflows minimize or eliminates manual tasks such as reconciliation of serious adverse events (SAE) or quality system product complaints with non-adverse events (NAEs), data queries, and re-entering of clinical data into safety applications. With an accessible and strong data foundation across the company, each organization can quickly complete tasks and make better and more informed decisions.
Easily Meet New or Changing Business Demand
Reduce level of effort to modify workflows and keep current with business processes
Many safety systems are often heavily customized. Whether on premise, or hosted and managed by a vendor, modifying processes require significant effort. Companies may introduce manual steps outside of the system to avoid making changes while still meeting new business requirements. Over time, there is a growing gap between what the business needs and the application delivers, creating significant inefficiencies and risk.
Designed for the life sciences industry, cloud-based pharmacovigilance solutions are enabling companies of any size to adopt enterprise-level safety applications. Business workflows are created and modified with configuration - not coding - reducing the administration burden and enabling safety organizations to be more agile in meeting new business and regulatory requirements.
Reduce overhead and validation to support new software releasesLegacy safety systems require significant resources and cost to upgrade to new releases. Small companies with resource constrained IT teams are often forced to outsource the safety database to a CRO or other service provider.
Modern safety applications leverage the cloud, enabling access to new capabilities through automatic updates several times a year. Leading industry cloud vendors also reduce the validation burden for customers by performing and documenting all elements of infrastructure qualification (IQ) and operational| qualification (OQ) for each major version. They provide sandbox and test environments, and user acceptance testing (UAT) scripts that can be leveraged for performance qualification (PQ).
Automatically scale as companies grow or expand into new markets
Pharmacovigilance solutions need to scale as companies evolve from clinical stage to product are cost prohibitive for smaller companies. Dedicated IT teams are also required to ensure adequate application infrastructure application and business continuity plans are robust.
Cloud safety solutions are delivered as a service. Biopharma companies do not need to worry about hardware, storage, or disaster recovery and the environment is continually monitored to ensure expected performance. There are also built-in gateways, including for U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), China National Medical Products Administration (NMPA), and Japan Pharmaceuticals and Medical Devices Agency (PMDA), that support regional submissions and make it easier for companies to expand into new markets.
Increase Business Process Outsourcing Flexibility and Value
Real-time data access for all parties for complete visibility and control Many emerging biopharma companies outsource the safety database to CROs / service providers and may not have direct access to the system - limiting visibility and control over the data. They rely on reports from CROs / service providers that can negatively impact decision-making if delayed or incomplete. With partners playing an increasingly critical role in pharmacovigilance, safety systems need to align with today’s business models.
Sponsor can easily access their data in cloud-based safety solutions - anytime, anywhere for oversight and operational visibility. They do not need to ask the CRO for a report, status of an adverse event, or current case processing throughput, resulting in cost-savings that can be reallocated to processing more cases. With real-time data access, there is greater alignment and more efficient collaboration between safety teams and partners. Data also becomes more accurate and higher quality when all parties are using the same safety solution and reviewing the same data.
Change Service Providers Without the Burden of Data Migration
As companies grow, they may change CROs / service providers to better align with evolving business needs. It is not uncommon to have data migration challenges, such as incorrect mapping of fields or incomplete data, when switching vendors - impacting reporting and other business milestones.
Cloud technology has lowered the barrier to owning a safety system. Providing a viable option to bring a safety system in-house, pharmacovigilance organizations can easily add or change CROs/ service providers, without the burden of data migrations and be more agile. Multiple CROs/service providers can also access the same safety system, while performing similar or different tasks.
With granular security and flexible workflows, pharmacovigilance teams can control what each partner can see and do in the system. For example, some service providers can process and review cases, while others can only view them without changing the record. Cloud safety solutions are designed to bring together different parties, supporting partner-defined workflows while keeping administration simple.
Reduce Training Time for Faster Onboarding and Better Adoption
One of the most important areas that impact training and user adoption is ease of use. Solutions that are intuitive from multiple perspectives - case processor, reporting team, or occasional user such as senior management who wants to look at an individual case safety report (ICSR), increases user productivity and adoption.
Built with a consumer web experience, cloud-based safety solutions require less training. End users can effortlessly navigate through the application to find information or complete tasks, and business administrators can quickly add users or modify fields. With an intuitive interface and a simplified environment—one unified safety solution - there are less errors and greater user productivity. Reducing onboarding and training time is also essential in today’s climate of higher employee turnover both internally and at partners.
Speed Of Safety Innovation
Legacy software vendors maintain multiple versions of safety solutions as customers are often several releases behind. For each version, the software vendor must spend resources fixing bugs as well as building, testing, and deploying security patches and integrations. With an ever-increasing portion of the development budget spent on maintenance, legacy software is slow to innovate and incorporate technology advancements, such as automation and artificial intelligence.
A true cloud solution only runs one software version for all customers, enabling more resources to focus on innovation and delivering new capabilities. They are also more robust as issues found by one customer are fixed for everyone. The pharmacovigilance solution continues to evolve with the industry, ensuring customers are always current with industry- driven best practices and regulatory requirements as well as incorporating new technologies to drive greater value.