Veeva CTMS
Improve Trial Visibility for Faster, Better Decisions
CTMS is an enterprise trial management system that provides end-to-end study management and monitoring capabilities for insourced and outsourced trials.
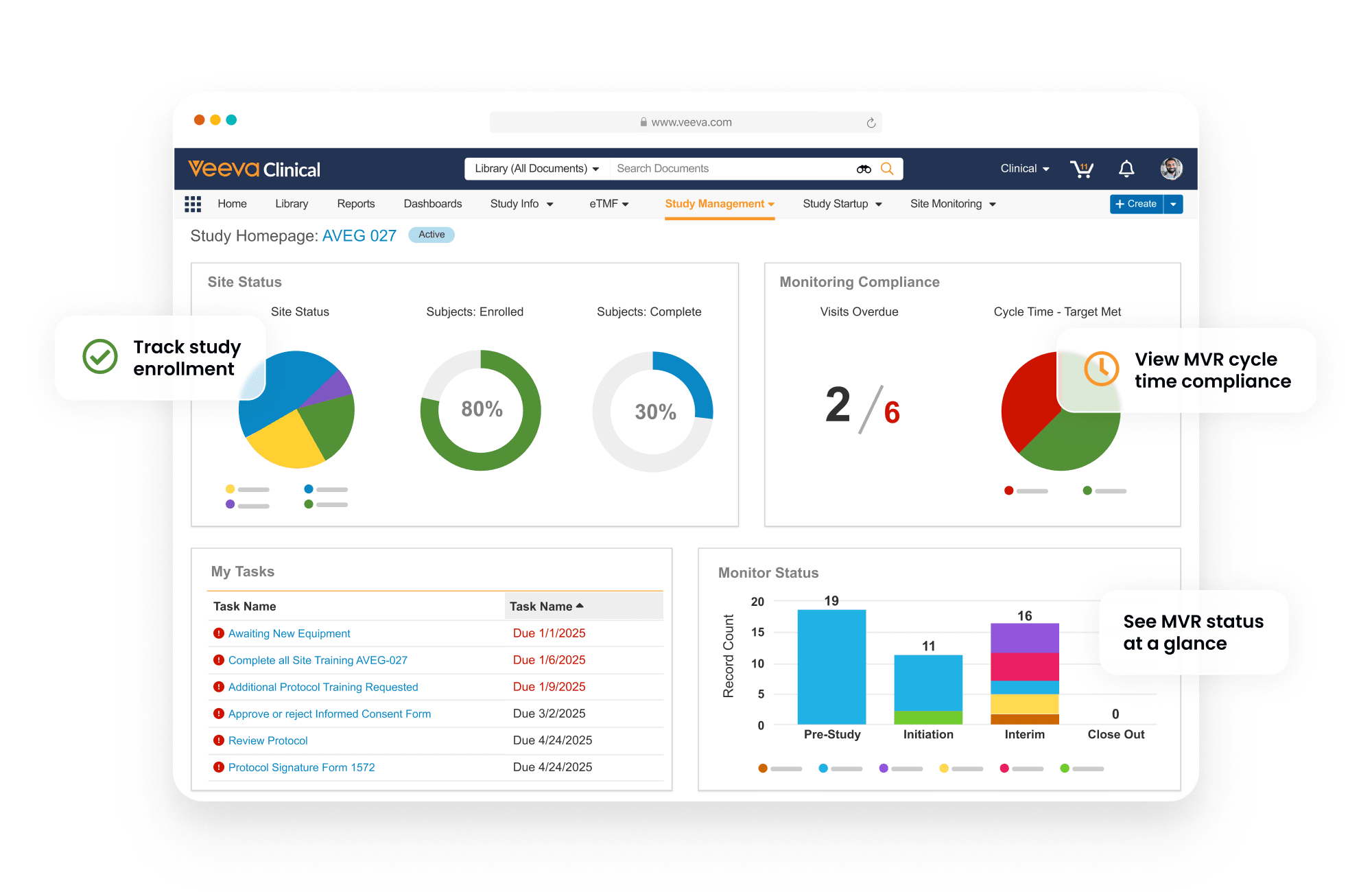
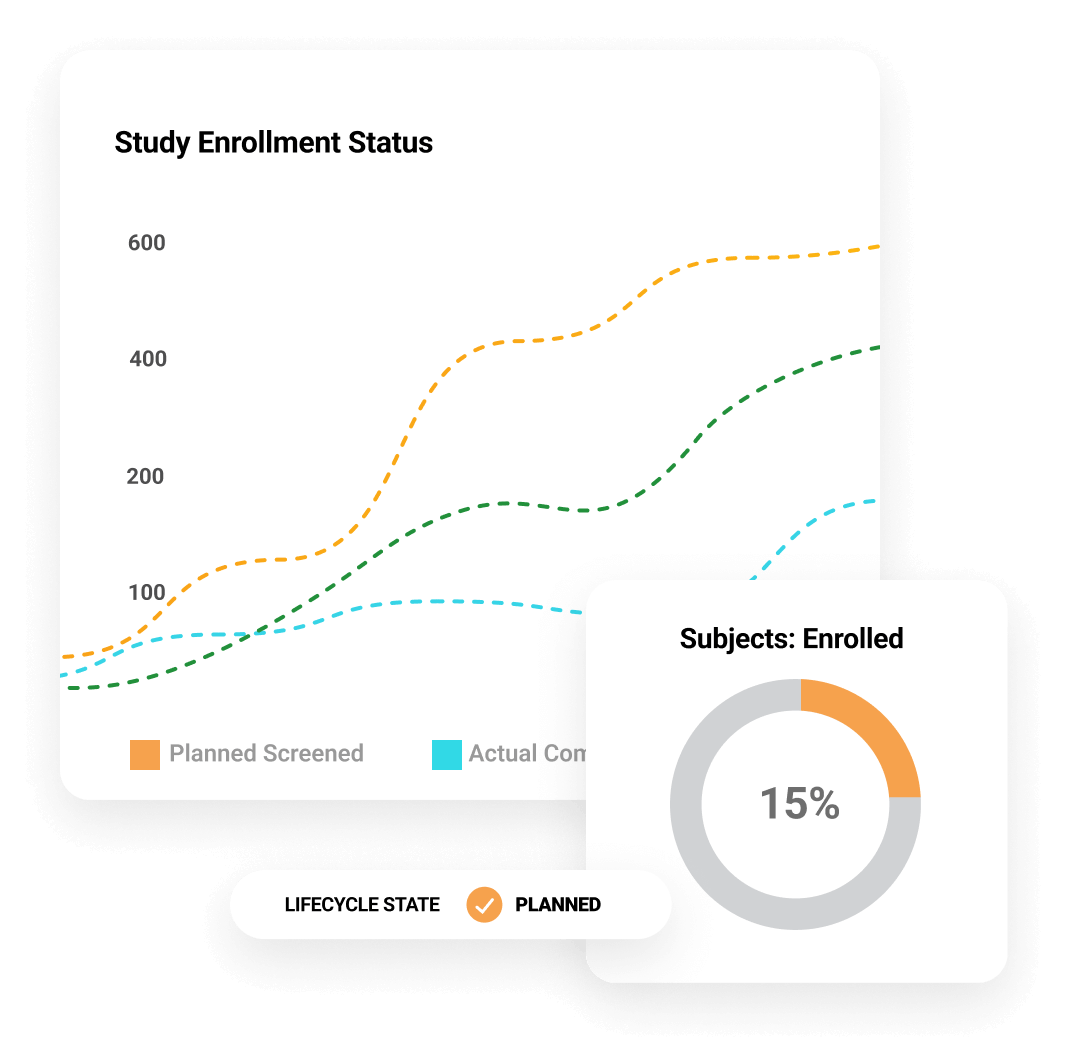
Dashboards and reports track key indicators, including enrollment and milestones, with drill-down to take action. Monitoring visit reports support automation and dynamic question branching. Trip reports are automatically filed within eTMF. Issues and Protocol Deviations are logged as needed and routed through resolution workflows to ensure closure. CTMS Transfer automates the daily transfer of data between CROs and sponsors using Veeva CTMS.
CTMS is connected with EDC to support enrollment, monitoring, payments, and navigation to casebooks directly from within CTMS. Investigator interactions synchronize with CRM for a 360-view.
Announced 2016 Status Very Mature Customers 100+
Find out why every biopharma needs a CTMS
Overview
Improve Trial Visibility for Faster, Better Decisions
CTMS is an enterprise trial management system that provides end-to-end study management and monitoring capabilities for insourced and outsourced trials.
Dashboards and reports track key indicators, including enrollment and milestones, with drill-down to take action. Monitoring visit reports support automation and dynamic question branching. Trip reports are automatically filed within eTMF. Issues and Protocol Deviations are logged as needed and routed through resolution workflows to ensure closure. CTMS Transfer automates the daily transfer of data between CROs and sponsors using Veeva CTMS.
CTMS is connected with EDC to support enrollment, monitoring, payments, and navigation to casebooks directly from within CTMS. Investigator interactions synchronize with CRM for a 360-view.
Impact
Centralize data and documents in one flexible system
30%
reduction in monitoring costs
50%
less time to author visit reports
80%
faster identification of issues
Why Veeva CTMS
Faster, higher quality trials
Customer Success
Streamlining trial management
for 200+ sponsors and CROs










Resources
Explore and Learn
View Features Brief
Veeva CTMS Features Brief

Read eBook
Discover Why a CTMS is Essential for Every Biopharma

Watch Customer Video
See How GSK Simplifies Site Monitoring

Watch Customer Video
See Why eTMF and CTMS are Better Together for Jazz

Read Case Study
Top 20 Pharma: CTMS Transformation Best Practices

Read Customer Story
Recordati Cuts Document Lifecycle Time by 25% Through Centralized Oversight on Veeva

Read Customer Story
CG Oncology Modernizes Trial Management Approach

Watch Demo
See How CTMS Transfer Works

Watch Video
See How Veeva CTMS Streamlines Trial Management