Quality products — those that fulfill their desired inherent properties and are suitable for their intended use — are the cornerstone of the biopharma and MedTech industries. But creating such products demands strict control of processes and procedures and adherence to many regulations.
To make this endeavor easier and optimize their quality operations, companies are seeking to remove siloes, create greater efficiency, and improve compliance across the value chain. Today’s advanced quality management software makes this possible.
How can advanced quality management software benefit biopharma organizations?
Advanced quality management software solutions are available to help support the full range of quality-related functions, including documenting and enforcing processes (through quality management systems (QMS), for example), managing regulatory content, training, validation and qualification, and managing lab data and operations (for instance, with laboratory information management systems (LIMS)).
With these solutions, biopharma and medtech companies can:
- Simplify processes by consolidating, connecting, and standardizing workflows
- Reduce errors by automating manual tasks
- Drive compliance and inspection readiness with enforced workflows, meticulous data tracking, and deeper data and process visibility
- Facilitate a quality-first culture by building quality into operations
The advantages of cloud-based quality management software
Companies often have the option of choosing on-premises or cloud deployment options for their quality software. In the former, software is installed on a company’s own hardware. In the latter, the software is stored and managed externally on cloud infrastructure, where customers simply access the software via the web.
Cloud-based deployment has several advantages:
Affordability
Cloud-based deployment typically involves a reduced upfront investment and eliminates the need for extensive on-site infrastructure and maintenance. Cloud-based quality solutions also benefit from multiple automatic and ready-validated updates, requiring minimal involvement from end users or in-house IT teams, further reducing costs.
The latest software, organization-wide
With a true cloud solution, all clients will be using the latest version of the software, meaning that companies can focus their time and efforts on innovating rather than maintaining multiple legacy software versions.
Faster deployment for a quicker go-live
Cloud-based solutions typically offer faster implementation times compared to on-premises systems, since customers won’t need to buy, install, and configure the right hardware, nor will they need to install the software locally. Customers can simply log in for immediate access.
Cloud-based quality management software can also feature built-in industry best practices that can further speed deployment.
Scalability
With cloud-based software, quality assurance (QA) and quality control (QC) teams can flexibly and quickly access greater computing resources to meet changing needs, making it easier to accommodate higher throughput, expand to new locations, or incorporate new functionalities.
Simple admin interfaces that offer easy no-code configurations also ensure cloud-based solutions can easily adapt to changing business needs.
What should you look for when selecting software to manage your quality processes?
Thousands of quality management software solutions exist today. But not all are created equal. To select the best option, companies should look for the following:
Cloud-based deployment options
Cloud-native software is often more affordable, faster to deploy, and easier to scale relative to on-premises solutions. Cloud-based quality management software also benefits from smooth automatic pre-validated updates, with minimal in-house maintenance required, meaning companies can focus on innovating.
A modern, intuitive user experience and automation
A modern user experience, enhanced with automation, is critical for improving system usability, facilitating better collaboration, improving visibility, and driving more productive workflows. The combination of intuitive interfaces and automated workflows reduces manual work, prevents errors and accelerates task completion. Thoughtful automation handles repetitive tasks while surfacing important decisions that need user attention.
A poor user experience and lack of automation can stymie system adoption and make simple tasks cumbersome, wasting precious time and resources.
Reporting and analytics capabilities
A modern, data-centric solution with reporting and analytics enables better decision-making. Data is used to improve visibility and in-application controls ensure compliance throughout the process. Dashboards and visual reports make data accessible for timely decision making, planning, scheduling, predicting events, and optimizing quality metrics.
Easy integrations with other technologies and equipment
A solution that integrates easily with systems and software applications can significantly reduce integration burden, as well as improve data flow, simplify processes, and drive productivity.
A data foundation for AI
Having a system that can offer a single repository of standardized high-quality data is critical for advanced analysis, automation, and for feeding into AI tools to help drive efficiency and productivity.
Excellent customer support and training
The success of any software deployment depends on the knowledge of the individuals using, maintaining, and administering it. It is therefore important to select software from a solution provider with the resources and expertise to troubleshoot any issues and effectively train and mentor your staff to ensure they can use, sustain, and evolve your software to meet changing requirements.
A solution provider with a reputation for excellence in the life sciences
A quality management software provider that specializes in the life sciences offers numerous benefits — from supporting global regulations and industry best practices to providing a fit-for-purpose solution for industry-specific workflows and being able to focus resources on more frequent innovations, to providing a community of experts for customers to tap into during and after implementation.
The future of pharmaceutical quality management: unified quality management platforms
Many quality management software solutions exist on the market. But deploying multiple fragmented point solutions, from different vendors, across your quality operations can pose challenges — from integration difficulties and higher training burden to greater resistance to adoption and more complex, error-prone workflows.
As such, companies are now turning to an emerging solution: software solutions that combine multiple quality management software applications on a single platform. By replacing multiple fragmented software solutions with a single source of truth for all quality information and data, companies could benefit from:
- Consolidated and consistent deployment, governance, maintenance, and support
- Faster user adoption and a lower total cost of ownership
- Easier integration across quality applications
- Less siloed working
- Connected business processes across systems and functions
- Simplified, standardized processes in corporate and local sites
- Greater efficiency and productivity with adherence to industry best practices
- Reduced cycle time for quality events
- Reduced audit risk and higher compliance
- Deeper and smoother collaboration internally and with partners and suppliers, throughout the value chain
- Continuous improvement and proactive optimization of quality
Veeva Quality Cloud: Unify QA, QC, and training in a single cloud-native system
What is Veeva Quality Cloud?
What is Veeva Quality Cloud?
Veeva Quality Cloud is a cloud platform that unifies applications, processes, and partners across content management, training, QMS, and QC lab solutions, helping accelerate the development and manufacturing of high-quality products. Quality Cloud comprises the following applications, which can be used alone or together within a single, unified platform:
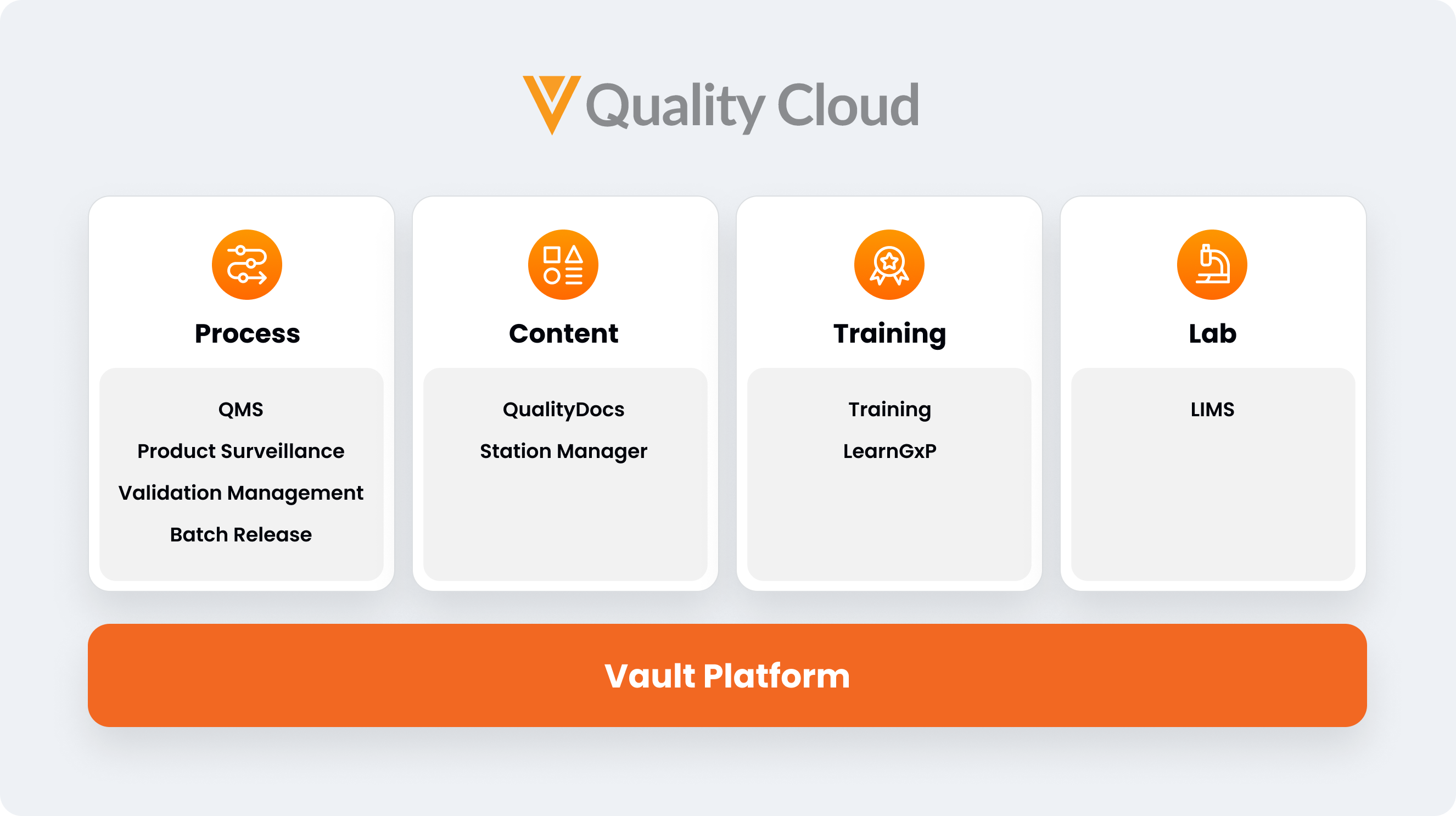
Process applications
Veeva QMS
Manages and tracks quality processes such as deviations, complaints, CAPAs, change controls, audits, and risk management
Veeva Validation Management
A paperless validation solution to optimize the end-to-end validation lifecycle
Veeva Batch Release
Aggregates data and content from QMS, LIMS, ERP, and regulatory systems to facilitate GMP release and market-ship decisions
Veeva Product Surveillance
Supports MedTech post-market surveillance processes and fully automated adverse event reporting to global health authorities
Content applications
Veeva QualityDocs
Gives greater visibility and control over regulated content and data across partners and all GxPs
Veeva Station Manager
A simple tablet-based application to ensure the right content is available 24/7 for manufacturing floor operators
Training applications
Veeva Training
A GxP-optimized learning management system (LMS) that manages authoring, approval, assignment, and assessment of training materials, in one place
Veeva Learn GxP
An eLearning library of accredited courses and microlearning videos to help organizations meet regulatory requirements and drive personnel development
Lab Applications
Veeva LIMS
A comprehensive modern cloud LIMS that optimizes life sciences QC by increasing right first time and connecting GMP quality business processes
How does Quality Cloud work?
How does Quality Cloud work?
Quality Cloud offers everything life science organizations need to manage their quality operations — all on a single platform:
- Processes: capabilities to standardize and simplify quality processes across sites and the value chain, ensure compliance with best practices, and manage and execute paperless validation
- Content: functionality to easily create, share, and manage (as well as automate the review, updates, and approval of) GxP documentation, and enable offline tablet or mobile access to quality docs on the shop floor
- Training: the ability to efficiently manage role-based qualification and training to drive audit readiness and regulatory compliance
- Lab: functionality to optimize GMP manufacturing quality control, managing everything from sample and program management to text execution, data review, and reporting
Deployed in the cloud, Quality Cloud applications are affordable, easy , quick to implement, can scale seamlessly with your needs, and receive continuous pre-validated updates.
Who Is Quality Cloud For?
Who Is Quality Cloud For?
Quality Cloud is ideal for biopharma, MedTech, and contract manufacturing companies seeking a modern, digital-first approach to quality management.
Quality Cloud is suitable for companies of all sizes — from emerging biotechs to large multinationals.
Various stakeholders from across your organization can benefit from Quality Cloud, including heads of quality and site QA, QA/QC managers, shop floor personnel, lab personnel, validation managers, and IT teams.
How does Quality Cloud help life science organizations?
Quality Cloud helps organizations accelerate the development and manufacture of high-quality medicines with a platform that unifies QA, QC, and training:
Remove operational silos
Modernize legacy systems by connecting, unifying, and managing all quality processes, content, events, data, processes, and applications within a single user-friendly and scalable cloud platform.
Simplify and standardize processes
Standardized processes — including CAPAs, change control, complaint handling, supplier management, lab investigations, training, and more — globally, for consistent and compliant quality operations that enable you to scale easily.
Create efficiency
Increase organizational efficiency with a modern digital system that eases user adoption, minimizes human errors, and deepens visibility into quality processes. Minimize time and effort by managing quality-triggered events and risk across quality disciplines, reduce process steps and workload with automation, and manage compliance with in-app controls.
Reduce cycle time
Reduce cycle time for quality processes and events — such as change control and batch release — with connected quality processes and visibility-enhancing reporting and AI capabilities.
Drive deeper collaboration across the value chain
Simplify collaboration with external partners with a single source of truth for quality data and content. Eliminate unnecessary back-and-forth and enable internal teams and partners to easily oversee everything from change controls and SCARs to supplier change notifications and more, through real-time access to content and automated workflows across applications.
Build a data foundation for analytics, automation, and AI
Attain a solid, standardized data foundation for advanced analytics, automation, and AI to unlock greater visibility and control of processes, make more timely decisions, optimize quality metrics, and drive proactive quality operations.
What sets Quality Cloud apart?
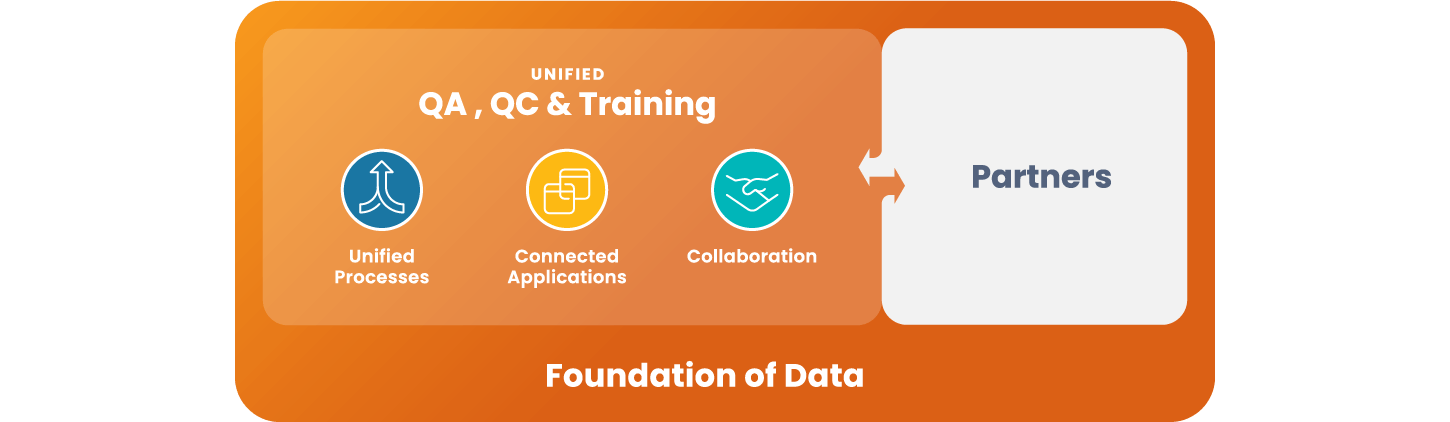
What sets Quality Cloud apart?
A unique ability to unify QA, QC, training, processes, and data
Veeva Quality Cloud is the only solution to unify all quality-related applications and processes in a single platform
Proven success in numerous transformational projects
We have delivered 25+ transformative global quality projects with Quality Cloud in the last 5 years
A transparent, agile approach to innovation
The Quality Cloud roadmap is closely aligned to customer feedback and requirements, with innovations delivered via a true agile process
Why Veeva?
Why Veeva?
- A global leader in cloud-based software for the life sciences
- Proven software solutions across the entire life sciences value chain
- Trusted by >1,000 customers — from the world’s largest biopharma companies to emerging biotechs
- Comprehensive customer support and training
“It is saving our analysts countless hours. It is saving my management team hours and driving a lot of benefits for our clients, who now have more visibility into their data and easier access to results.”
Marcus Massingham
Senior Director, Quality Systems, GSK
Learn More
“With Veeva Quality Cloud, we now have the quality systems in place to easily scale our manufacturing operations while maintaining compliance across stakeholders.”
Sérgio Simões
Vice President, Bluepharma
“Partnering with Veeva and having help with validation, plus taking out-of-the-box-type functionality allowed us to implement far faster than almost any other product out there.”
Greg Adcock
Senior Director, Quality Systems, Arcellx
“Having everything in one place was fantastic. In addition, the response time of the technology is very good, and the single sign-on is much appreciated by end users.”
Letizia Caccialupi
Global Head of CHC Quality Systems and excellence, Sanofi Consumer Healthcare
“Modernizing quality is a lever enabling our company transformation.”
Maité Durrenbach
Chief Quality Officer, Sanofi
FAQ
Quality management software is software that helps organizations maintain stringent quality standards. Such software spans a wide variety of quality-related tasks, procedures, and processes, and includes quality management system (QMS) software, content management systems, training software, and laboratory information management systems (LIMS).
Many life sciences organizations have a fragmented landscape of disparate quality management software applications. However, quality management software solutions are now available that bring together all quality-related applications, data, processes, and partners on a single software platform to drive efficiency and collaboration.
A quality management system (QMS) is a structured framework of processes and procedures that help organizations consistently meet customer requirements and maintain quality standards. A QMS encompasses policies, documentation, and activities that ensure products or services are delivered effectively while promoting continuous improvement throughout the organization.
QMS software is a digital platform that automates and streamlines quality management processes. It helps organizations manage documentation, track compliance, monitor workflows, conduct audits, and handle quality-related tasks. The software centralizes quality data and provides tools for reporting, analysis, and continuous improvement activities.
Veeva Quality Cloud is a cloud platform that unifies applications, processes, and partners across content management, training, QMS, and QC lab solutions, helping accelerate the development and manufacturing of high-quality products. Veeva Quality Cloud comprises a suite of nine applications which can be used alone or together within a single, unified platform. Discover more about Veeva Quality Cloud.
No, Quality Cloud offers much more than just QMS software. Quality Cloud is a cloud platform featuring applications spanning the entirety of quality processes, content, training, and lab data. Quality Cloud comprises 9 quality applications in total, each of which can be used alone or together within the same application space:
- Veeva QMS
- Veeva Validation Management
- Veeva Batch Release
- Veeva Product Surveillance
- Veeva QualityDocs
- Veeva Station Manager
- Veeva Training
- Veeva Learn GxP
- Veeva LIMS
Discover more about Veeva Quality Cloud applications today.
Quality Cloud helps organizations accelerate the development and manufacture of high-quality medicines. As an intuitive, cloud-based platform that unifies QA, QC, and training, Quality Cloud can help life sciences teams remove siloes, simplify and standardize processes, minimize errors, reduce cycle time, and drive deeper collaboration across the value chain. What’s more, Quality Cloud can provide a strong foundation for advanced analytics, helping to drive a more proactive approach to quality operations.
We built the Quality Cloud and applications to meet the specific content and data management needs of the life sciences industry, including pharmaceutical, biotechnology, medical device, and diagnostic organizations. GxP rules — such as FDA 21 CFR Part 11 and EU Annex 11 — were taken into consideration and implemented into the product design, including comprehensive audit trails, electronic signatures, and many other features. Applications are subject to validation activities (installation qualification (IQ)/operational qualification (OQ)) that can be leveraged by customers, streamlining customer validation efforts.
Integrations are supported by open, representational state transfer (REST)-based APIs documented at https://developer.veeva.com/. REST supports several integration tools (middleware such as Mulesoft, Tibco, and Informatica, etc.) or point-to-point interfaces (Java, C#, Python). Customers can leverage architectures that fit the overall integration landscape (point-to-point, staging databases, transactional ODS, data lake, etc.).
Ease of external system integration will depend on the external system’s integration capabilities, since Quality Cloud has a well-established integration approach. For example, an external system with a well-developed, open API is typically easier to integrate with Quality Cloud.









