Blog
The How-to’s Behind Agile Design at Veeva R&D Summit
Sep 26, 2019 | Karin Ondricek
Sep 26, 2019 | Karin Ondricek
People come to Veeva R&D Summit because they want to change. They want to improve some problematic process and know that Summit is a place to get actionable advice. That was surely true in the Vault CDMS track this year. Speakers shared their stories of accomplishment and the audience responded with questions about how. “How do you get sites to enter data in two to four days? How do you enforce compliance with your case report form (CRF) standards? How did you know the CRFs were correct if you didn’t have a spec?”
The answers to “how” inevitably touched on process and technology. Christopher Otto from Eli Lilly commented on how you cannot swap Vault EDC into an existing process–many elements are different and people must leverage the new capabilities to achieve the desired efficiencies. He shared that developing a new process model was one of Lilly’s top priorities as part of planning for a broader roll-out of Vault CDMS.
Speakers shared technology and process changes across many aspects of an EDC study build:
- Spec-less design – Harbal Sidhu from ICON explained how Veeva’s interface for building studies is easy enough that someone with clinical expertise can design forms straight from the protocol and programming staff are only needed for writing complex edit checks.
Jennifer Nezzer from Lotus Clinical Research shared that spec-less design saves them weeks of time previously spent building and reviewing a spec. Instead of comparing spec to protocol and EDC to spec, they now compare the EDC (blank or annotated CRF) directly with the protocol.
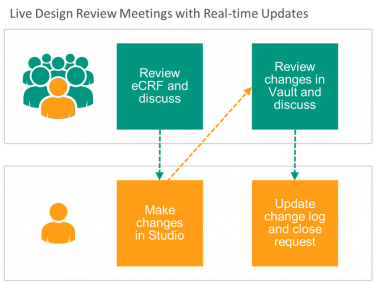
- Real-time updates – David Templeton from Penumbra spoke about the importance of having a framework for decision making during the design reviews. He noted how easy it is to make changes during the design reviews and advised others to be thoughtful regarding which decisions to make in real-time and which to discuss later with other study teams.
- Collaborative configuration – Janine Del Vecchio from Insmed explained how the close collaboration with Veeva continued beyond the design reviews. As Veeva’s configuration specialist completed each update, the Insmed team would review the change in a shared development environment and provide approval or feedback in the online tracking sheet. The collaborative process was so effective, their UAT surfaced only 32 findings and was completed in seven working days.
- User acceptance testing – Vikas Gulati from Vertex Pharmaceuticals Inc. spoke about how dramatically their UAT process changed. Historically, their UATs would entail multiple weeks of back-and-forth with their vendor; whereas their latest UAT with Veeva was completed in only two days. With the introduction of Veeva’s auto-generated report documenting the differences between studies, Vertex is now updating their process to eliminate UAT requirements for forms and fields previously tested for their template study.
- Standards re-use – Michelle Harrison, also from Vertex, shared that over 50% of the CRFs in a recent study were re-used as-is, with zero changes. In their primary therapeutic area, Vertex’s goal is for 75% of the CRFs in a study to be from their standards without changes. Harrison described the rigorous governance that Vertex put in place to maintain their standards and enforce compliance. Many in the audience were clearly impressed, peppering Vertex with questions about their processes and recipe for success.
- Consistency without standards – Evelyn Dorsey from Cara Therapeutics provided the perspective of a pharma without formal standards. She pointed out how using Vault CDMS with different CROs for different studies created greater consistency in their CRFs and for downstream programming. Previously, each CRO had their own way of collecting data. Now, Cara has greater consistency without a formal standards library.
Veeva R&D Summit is about more than technology. It is a place where people learn new ways of working. It is a place for sharing first-hand experiences and advice for making things better. We are excited that data management teams left talking about renovating the processes for building studies in an EDC.
If you missed this year’s event, you can read about Veeva’s live UAT meetings with real-time updates, one of the many process and technology innovations described by Vertex, Lotus Clinical Research, and others.
Join Veeva Summit to see new trends and best practices in training and quality management.