Blog
Global Approach to Core Packages: Simplify to Speed Up Submissions and Variations
Mar 28, 2024 | Paul Attridge and Kim Brownrigg
Mar 28, 2024 | Paul Attridge and Kim Brownrigg
Changing regulations, supply chain requirements, and challenges with staff continuity make local submissions preparation increasingly complex. Left unaddressed, this will lead to longer preparation timelines and more work for regulatory teams.
There will always be a need for localized submission dossiers. Even submissions that apply to several markets require local content and generate specific health authority (HA) questions, which result in divergence from the core dossier. Nevertheless, by automating the dispatch and update cycles from core packages to local submissions, regulatory teams can achieve faster lifecycle variations.
Traditional formats aren’t effective
The core package format describes a set of documents prepared by headquarters and used in local market submissions. Initially intended to streamline and accelerate submission preparation, most formats used today result in tradeoffs between global and local teams.
Core packages typically appear in two styles — and neither is perfect. The first is a comprehensive dossier based on the framework of a specific HA (usually in the U.S. or EU), which is adjusted for local divergence. Although this package is easier for global teams to manage, it’s a heavier lift for the local affiliates responsible for reviewing content and submissions: they are required to deliver against specific local content requirements despite only globally common elements being available to them.
The second style creates a core package for a subset of countries with similar requirements and links to content in common. Although it involves less effort for local offices, this approach quickly creates headaches for global teams, which monitor and adjust to changing local regulations.
A hybrid core package approach that combines the positive aspects of each format can simplify review and approval cycles. For this to happen, global teams would create a single core package that ingests registered content, and then dispatch market-relevant documents for review [Figure 1].
Figure 1: Requirements for good core package management

Bringing global and local closer together
Rather than sequential processes, a more effective alternative is for global and local teams to work in parallel [Figure 2].
Following this approach, headquarters assesses submission documentation upfront and creates the global content plan before sharing it with local affiliates for review. Once received, local teams fill in missing information, prepare local documents, and initiate translations. Meanwhile, headquarters finalizes global documents (e.g., by pulling in registered documents into content plans) before notifying local offices that approved content is ready and dispatching it.
Figure 2: Keeping parallel processes on track
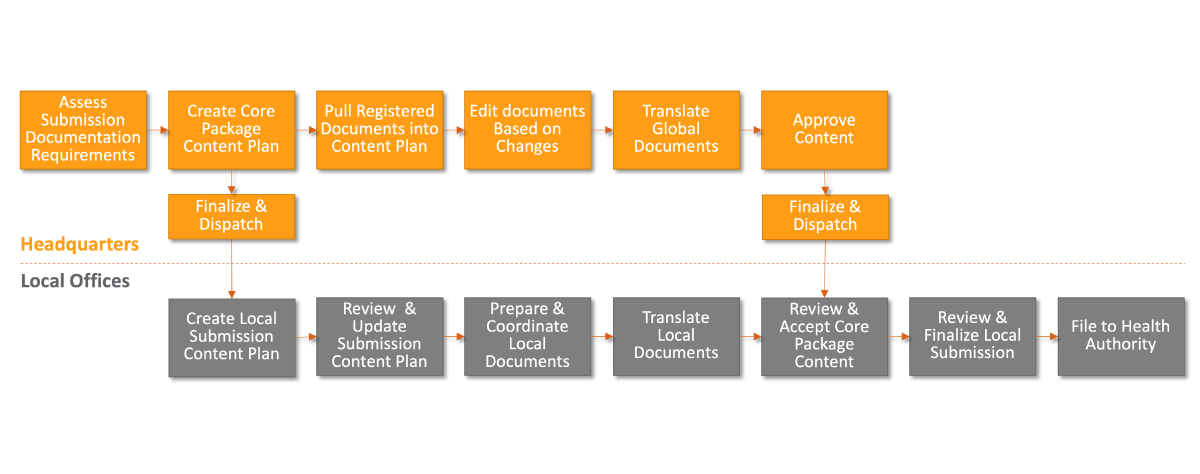
Source: Veeva Business Consulting
Local affiliates — whether outsourcing partners or internal regulatory teams — waste valuable time if required to locate and work with the correct document version. Technology underpins effective global-to-local collaboration by alleviating local teams’ workload as they prepare, distribute, and submit to HAs.
When global and local teams use Veeva Vault RIM, they can:
- Develop content plans more efficiently. Global content plans are generated automatically from templates, and include placeholders, while content is collected from different markets.
- Ensure requirements are met on time. Section owners are assigned responsibilities and due dates, while real-time tracking means it’s easy to identify which information is missing.
- Identify changes in registered content. Features such as document auto-matching help teams locate approved content or lock specific document versions. Relevant groups (e.g., clinical, medical writing) view and use content in a controlled environment.
- Share specific documents or sections. Local teams can partially or fully preview global content plans, which are dispatched iteratively.
- Make comparisons with submission content plans. Submission content plans are automatically created from (and can be compared with) global content plans. Local teams can accept or reject proposed updates.
Simplify with each cycle
As organizational understanding grows, global and local teams generally go through fewer review cycles. This means that despite regulatory complexity, the processes underpinning submissions will simplify over time — leading to faster submissions and fewer HA questions.
Register to attend Veeva R&D and Quality Summit, North America, to learn more about regulatory transformation.