Veeva PromoMats
Accelerate the End-to-End Content Lifecycle
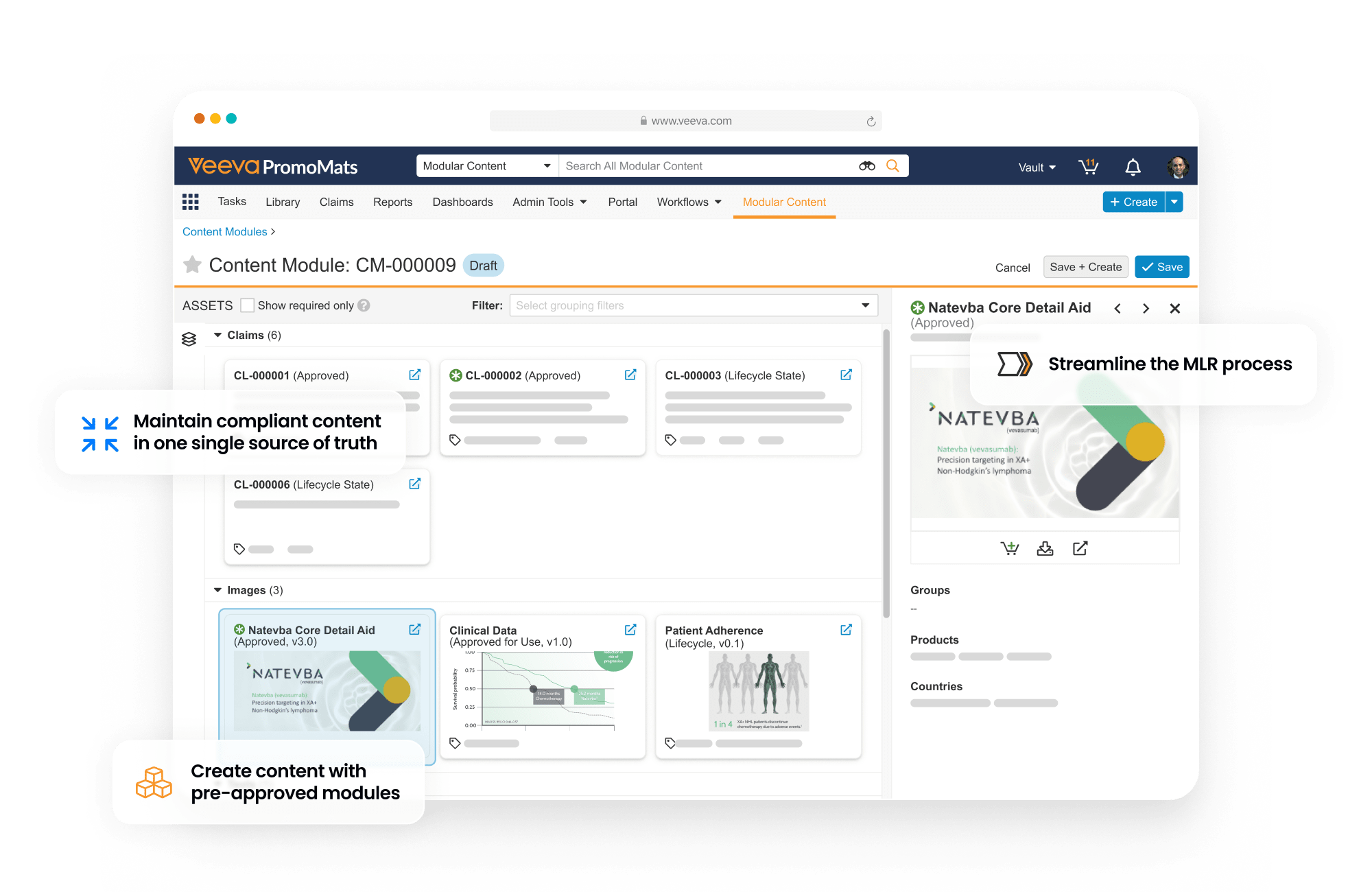
PromoMats is a regulated content management application that supports the full lifecycle of promotional content. It enables content creation, review and approval, digital asset management, claims management, and modular content.
Connected with CRM, PromoMats provides automatic distribution of promotional content via applications like CLM and Approved Email. Veeva’s Open API allows for integration with third-party systems such as web content management or campaign management applications.
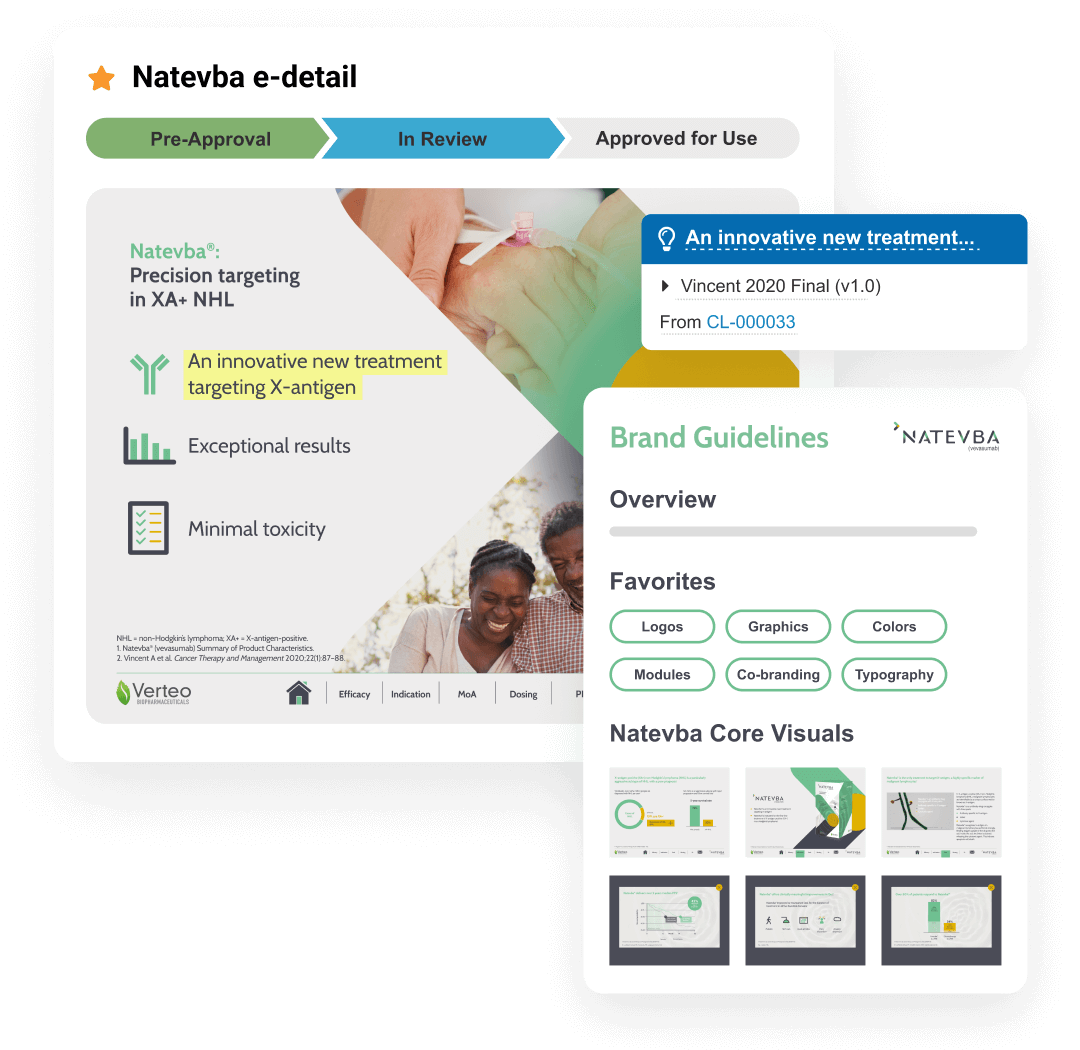
This application includes Brand Portal, a central repository that makes content easily accessible for internal users while enhancing content reuse. PromoMats automatically generates eCTD compliance packages for post-marketing and pre-clearance submission to the FDA.
Announced 2011 Status Very Mature Customers 100+
Explore the latest innovations
Overview
A Flexible, Scalable Solution
for Commercial Content
Compliant content at scale
PromoMats is a regulated content management application that supports the full lifecycle of promotional content. It enables content creation, review and approval, digital asset management, claims management, and modular content.
Connected with CRM for automatic distribution
Connected with CRM, PromoMats provides automatic distribution of promotional content via applications like CLM and Approved Email. Veeva’s Open API allows for integration with third-party systems such as web content management or campaign management applications.
Single source of truth for all your content
This application includes Brand Portal, a central repository that makes content easily accessible for internal users while enhancing content reuse. PromoMats automatically generates eCTD compliance packages for post-marketing and pre-clearance submission to the FDA.
Impact
Transform the content lifecycle
+50%
average increase in speed to market
+40%
growth in content reuse with modular content
+20%
reduction in cost to create content
Why Veeva PromoMats
Speed compliant content at scale
Customer Success
Trusted by 450+ top and
emerging biopharmas globally


















Resources
Explore and Learn
Read Features Brief
Veeva PromoMats Features Brief

Watch Video
Pioneering MLR Transformation with Veeva AI

Read Features Brief
Veeva AI for PromoMats

Learn More
Latest Innovations from 2025

Read White Paper
Building a Compliant Content Foundation for the Future

Learn More
2024 Innovation Guide

Read Case Study
Roche: Why Pharma Needs a Global-to-Local Content Strategy

Read Case Study
Bristol Myers Squibb: Building an Omnichannel Content Strategy
