Adoption of Veeva Vault Quality Applications Grows as Industry Moves to Unify Processes Across Global Stakeholders
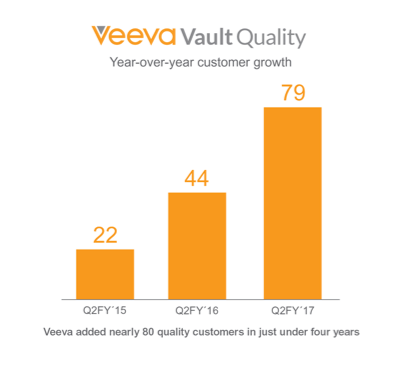
Veeva adds nearly 80 Vault Quality customers,
including four of the top 20 global pharmaceutical companies
PHILADELPHIA – 2016 Veeva R&D Summit – Oct. 18, 2016 – Today at the Veeva R&D Summit, to an audience of more than 600 industry leaders, Veeva Systems (NYSE: VEEV) announced broad industry adoption of Veeva Vault QualityDocs and Veeva Vault QMS as the life sciences industry focuses on unifying quality management. Prompted by the need to seamlessly bring together quality work processes and content management, Veeva has added nearly 80 quality customers in less than four years and eight customers have signed on for Vault QMS since the product was released four months ago.
Four of the top 20 largest pharmaceutical companies are using Veeva Vault quality applications to drive greater global alignment, collaboration with external contract manufacturers and partners, and harmonization of quality processes. With Veeva, companies get a modern cloud solution to standardize the flow of information and support multiple, concurrent outsourcing models, while ensuring process transparency.
In 2015, the FDA issued warnings to 10 drug companies for data integrity violations – the most in a decade. 1 A mix of disparate legacy systems add to data integrity challenges by fragmenting internal processes and making it harder to incorporate global partners. With increased outsourcing of quality manufacturing and pressure to adhere to manufacturing regulations, full visibility into quality processes and supply chains is becoming more critical.
“Collaboration and access across global stakeholders are not only critical for achieving compliance, but also to ensure data integrity,” said Craig Gassman, associate director of regulatory operations at Karyopharm Therapeutics. “With Veeva Vault Quality, all of our partners have real-time visibility in the cloud and we gain end-to-end control of critical documents and processes.”
“The demand for Veeva Vault quality applications is indicative of the industry need to unify quality processes and documentation across business units and borders,” said Mike Jovanis, vice president of Vault Quality at Veeva. “Vault’s true multitenant cloud architecture connects quality-related processes, documents, and stakeholders for better visibility and collaboration throughout the entire global supply chain.”
Vault QualityDocs and Vault QMS is the only integrated suite of quality applications for seamless end-to-end content and quality management. Both applications are built on Veeva Vault, a proven cloud platform and suite of applications that provide a single source of truth across the enterprise. Native interoperability between Vault applications empowers life sciences companies to streamline their business processes across an increasingly broad ecosystem of internal and external stakeholders.
In other news today, Veeva announced that ICON is streamlining its regulatory and quality operations with Veeva Vault applications, and that inVentiv is expanding its adoption of the Veeva Vault Clinical Suite to expedite site activation for clients. Read our press release for ICON and our press release for inVentiv to learn more.
Additional Information
For more on the Veeva suite of quality products, visit: veeva.com/quality
For more on Veeva Vault QualityDocs, visit: veeva.com/QualityDocs
For more on Veeva Vault QMS, visit: veeva.com/QMS
Connect with Veeva on LinkedIn: www.linkedin.com/company/veeva-systems
Follow @veevasystems on Twitter: www.twitter.com/veevasystems
Like Veeva on Facebook: www.facebook.com/veevasystems
About Veeva Systems
Veeva Systems Inc. is a leader in cloud-based software for the global life sciences industry. Committed to innovation, product excellence, and customer success, Veeva has more than 450 customers, ranging from the world’s largest pharmaceutical companies to emerging biotechs. Veeva is headquartered in the San Francisco Bay Area, with offices in Europe, Asia, and Latin America. For more information, visit www.veeva.com.
Forward-looking Statements
This release contains forward-looking statements, including the market demand for and acceptance of Veeva’s products and services, the results from use of Veeva’s products and services, and general business conditions, particularly in the life sciences industry. Any forward-looking statements contained in this press release are based upon Veeva’s historical performance and its current plans, estimates, and expectations, and are not a representation that such plans, estimates, or expectations will be achieved. These forward-looking statements represent Veeva’s expectations as of the date of this press announcement. Subsequent events may cause these expectations to change, and Veeva disclaims any obligation to update the forward-looking statements in the future. These forward-looking statements are subject to known and unknown risks and uncertainties that may cause actual results to differ materially. Additional risks and uncertainties that could affect Veeva’s financial results are included under the captions, “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” in the company’s filing on Form 10-Q for the period ended July 31, 2016. This is available on the company’s website at www.veeva.com under the Investors section and on the SEC’s website at www.sec.gov. Further information on potential risks that could affect actual results will be included in other filings Veeva makes with the SEC from time to time.
###
Contacts:
Veeva Systems
pr@veeva.com
1 (PwC: March 2016), Data Integrity Problems a Growing Risk to Global Pharma Companies