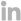Fragmentation
To meet the obligations of globalization and IDMP, regulatory must address one of its primary challenges—system fragmentation.
Fragmented system landscapes contribute to process delays and a lack of visibility. Depending on the size of your company, an individual process may touch five to 95 different systems and spreadsheets—with correspondence, commitments, submission documents, and archived dossiers each managed independently. Companies may have separate tracking spreadsheets for each step in the process and for each product—resulting in hundreds of individual trackers. As the number of systems grows, the process becomes more manual and inefficient.
There are many places to manage information
...yet it is almost always done in Microsoft Excel.
Processes and system environments are also fragmented by region. Headquarters lacks sufficient visibility into local activities when regional teams work within their own systems. Without a shared central system, managers are forced to request status updates and assemble reports manually, which increases errors and delays.
Inefficiency
Inefficiency is a second internal driver behind regulatory leaders’ desire for transformational change.
Most regulatory activities are effective in terms of compliance, yet score low on efficiency. According to a 2016 RIM industry report, the average efficiency rating across 17 regulatory capabilities is 39%.iii Given that compliance is regulatory's primary function, it is not surprising that few organizations focused on regulatory's efficiency. Most companies that measure RIM performance have low confidence in their metrics. That may change soon. The majority of companies surveyed plan to start measuring RIM performance within the next 12 months.
Another efficiency indicator is the time it takes for a regulatory team to answer basic questions such as "What is the status of a label change in an affiliate office?" or "What is the status of our product renewals by country?" Half of the surveyed companies took 'days or weeks' to provide accurate answers, as opposed to hours or minutes.
As companies grow, so can the severity of the problem. One top 20 pharma reported spending five weeks to perform a single registration impact assessment for a manufacturing change. Another firm estimated wasting 75,000 hours per year on manual data entry and rework due to inefficient change control and variation management processes.
Inefficiency Problem

Many have already set priorities and begun executing plans to transform regulatory’s operations. Read next chapter