Cara Therapeutics ran a Phase II randomized study with a protocol typical of the increasingly complex, data-driven approach that the modern clinical trial landscape demands. Thanks to an integration between two best-in-class vendors in EDC and IRT, Cara easily made mid-study changes and managed clinical data.
Challenges
Time Constraints
The company’s fast pace as a clinical-stage biopharmaceutical company meant strict deadlines. Cara’s goal was to use Phase I results collected in the first quarter to inform protocol design and begin implementation of their Phase II trial by the end of the second quarter.
Protocol Design Complexity
Their Phase II study for a novel antipruritic drug required a system capable of keeping up with its inherently complex protocol. The study utilized stratified randomization to distribute chronic kidney disease patients across three treatment arms and one placebo.
It capped enrollment of patients in certain strata according to percentages in each treatment arm. Stratification and enrollment were both heavily dependent upon data collected during a seven-day run-in period in which patients were screened according to severity of pruritus and severity of chronic kidney disease.
Cara’s protocol also allowed patients to be rescreened. They needed a system that would help them keep track of patients to determine if they were unique or rescreened.
Mid-Study Changes
Their protocol required flexibility, as it shifted gears mid-study to drop insubstantial treatment arms. It called for an interim assessment of patients once enrollment rates fell to 50 percent due to subject dropout and completion. This was to determine if any treatment arms no longer needed to be assessed due to emergent trends in the data or lack of substantial patient numbers in one or more of these dosage categories. They also needed to change enrollment cap percentages based on stratification of patients on dialysis and those who were not.
Solution
Cara Therapeutics utilized an integration between Veeva’s Vault Clinical Data Management System (CDMS) and Suvoda’s IRT for their study to address these challenges. The result was a fast, flexible solution that simplified data capture and management at all stakeholder levels, offered predictability in the midst of dynamic, complex protocols, and mitigated risk in avoiding human error common in data entry across disparate systems.
Simplicity
Cara Therapeutics needed a simple solution that allowed them to manage data across IRT and EDC systems. More specifically, they needed coordinated systems to properly screen, stratify, and randomize patients, to direct and monitor supply for multiple drug dosages, and to properly monitor and limit enrollment. With Suvoda and Veeva’s integration in place, Cara managed and monitored all these processes with a cohesive integrated solution.
By predefining data standards, Veeva and Suvoda’s teams were also able to help Cara avoid errors in data entry and eliminate the time it takes to reconcile disparities or duplicate data points. As a result, Cara’s team of stakeholders were able to mitigate risk associated with data errors common to working across multiple systems.
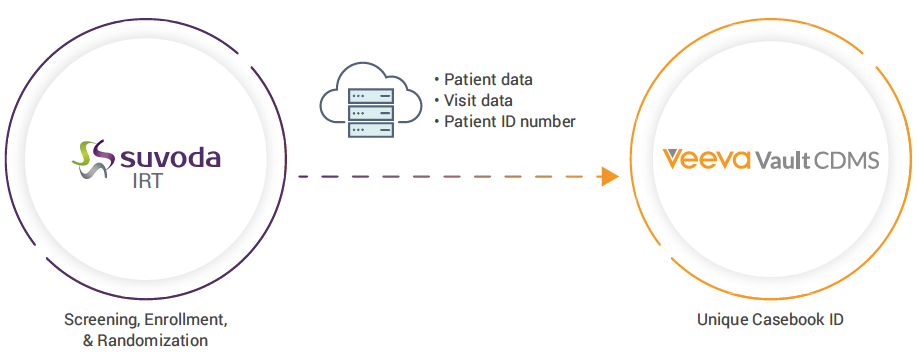
Streamlined IRT & EDC Integration for Cara Therapeutics
“This integration created simplicity at every level, removed manual data entry, and brought cohesiveness to all the stakeholders involved in our program.” Catherine Munera, Ph.D., Cara Therapeutics
Speed
Typically, a multi-vendor integration can lengthen the time to study startup. Veeva and Suvoda were able to standardize data points across both their EDC and IRT systems to shorten this initial setup period for Cara and employ the integration for additional studies in their pipeline.
The solution also saved the study team time with data entry. Cara’s site users only had to enter information once in Suvoda IRT and the integration populated patient and visit data in near real-time to Vault EDC. Additionally, this data was saved in both systems and was reused rather than re-entered if a patient initially failed screening and was later rescreened.
Flexibility
A primary complaint for study stakeholders using EDC and IRT systems is that mid-study change orders are slow and complicated. Veeva and Suvoda anticipated potential change scenarios in their integration to eliminate the need for most change orders and drastically reduce mid-study change timelines.
Thanks to the flexibility between Veeva’s EDC and Suvoda’s IRT system, changes to enrollment caps, patient re-enrollment, dropping treatment arms, and other unplanned study changes were easily and quickly implemented with few or no change orders involved. A well-implemented and flexible integration offers a degree of predictability that, coupled with responsive support teams, allows sponsors like Cara to move from study to study without worrying what their EDC and IRT systems can and cannot handle.
The Takeaway
Veeva and Suvoda’s integrated IRT and EDC solution provides the functionality and advances of two best-of-breed technologies without sacrificing the desired simplicity of working with a single vendor. After experiencing the simplicity, speed, and flexibility of this integrated solution, Cara Therapeutics leveraged the two systems for several subsequent studies.
By providing seamless eClinical solutions to handle the increased complexity in the clinical trials landscape, Veeva and Suvoda are working to help sponsors focus on delivering treatments to patients.
More Customer Stories
Explore the Strategy
See Vault CTMS in Action
Explore Site Benefits



