Blog
Plan for eCTD 4.0 and Improve Submission Success
Jul 25, 2024 | Rich Merrick
Jul 25, 2024 | Rich Merrick
Universal global deadlines for using eCTD 4.0 are a few years away. However early preparation is key. Veeva Vault RIM’s data model is evolving to support users with ICH’s latest e-submission specifications.
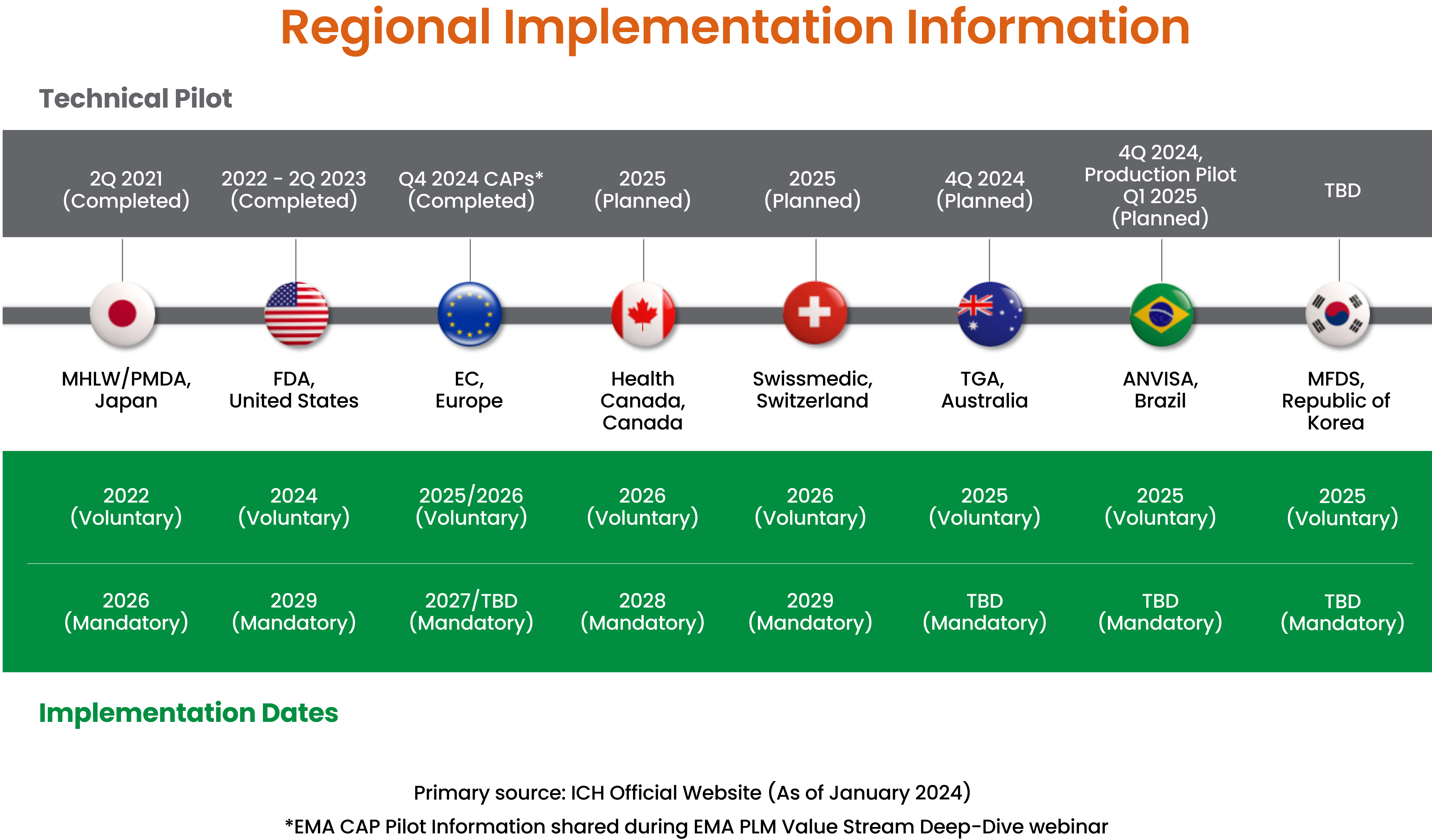
A new ICH Version (1.6) was recently published in May 2024, and regional implementation guides are being finalized. In Japan, eCTD 4.0 is already available to submit new product applications, and more global regulatory agencies are defining mandatory dates for eCTD 4.0 (Figure 1). Japan’s PMDA has set 2026 as a deadline for mandatory submissions, while the FDA has updated implementation guidance and set the deadline for 2029. Proactive planning is essential, since eCTD 4.0 incorporates a number of new concepts that differ significantly from previous versions. Regulatory leaders will benefit from diving into the specification and regional guidance, and determining how it will change practices within their companies and teams.
Figure 1. eCTD 4.0 Implementation Schedule
A technological advancement
eCTD 4.0 represents the latest evolution of an ongoing journey to make the global submission and review process more efficient, consistent, and user-friendly for publishing teams and reviewers alike. First introduced in 2002, eCTD revolutionized regulatory processes by eliminating the need for multiple paper dossier copies and manual review of paper stacks. By 2018, the FDA required electronic submissions, and the eCTD format had been refined, updated, and quickly adopted by many biopharma companies.
Although some observers have argued that the new eCTD 4.0 fails to use more modern, data-based approaches with capabilities that already existed with eCTD 3.2.2, the update still presents progress and introduces new features and enhancements.
Simplified global standard
While eCTD 3.2.2 provided a global standard format, there were still country nuances such as the use of node extensions or Study Tagging Files (STFs). Providing the ability to submit in one standard format across all global regions, eCTD 4.0 harmonizes a global approach by using a single submission unit XML and introducing controlled vocabularies for all regions. Regional document type definitions (DTDs), which defined XML structure and other features in eCTD 3.2.2, are no longer required.
The changes result in more consistent processes for regulatory activities. By using the same schema from region to region, it is easier for users to reuse content. There are fewer manipulations to the structure to ensure it will be accepted by the target country. eCTD 4.0 also introduced the “controlled vocabulary” approach that ensures updates to the regional specifications can be made without major changes to the underlying schema. Sponsors must rethink how they submit and enrich data in existing systems to prepare for eCTD 4.0.
While it was possible to reuse data in eCTD 3.2.2, eCTD 4.0 encourages leveraging documents through unique identifiers. Systems can now calculate if a document was already sent and automatically write the XML to contain the unique identifier to exclude the document from the submission package. This also means the agency doesn’t need to re-review the same document multiple times, resulting in a more efficient process. Without good document management, customers must manually track document distribution. Unified publishing and document management systems can significantly streamline this process by providing seamless end-to-end tracking and visibility into the status for all documents.
“Forward compatibility” in eCTD 4.0 allows reusing or referencing documents in eCTD3.2.2 submissions. Systems will need to support all regional and ICH versions of eCTD to ensure an accurate lifecycle.
Lifecycle changes
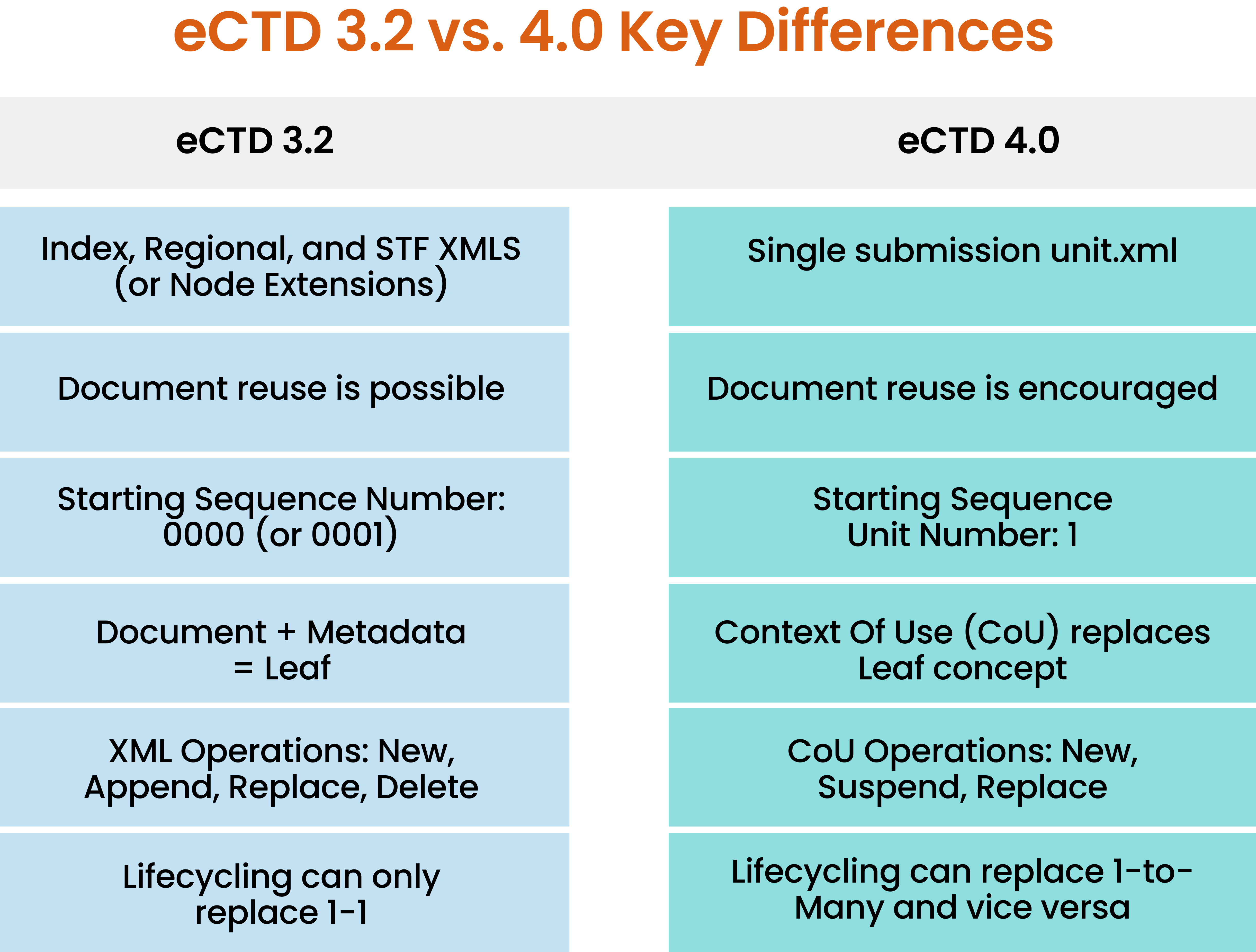
eCTD 4.0 replaces the ‘leaf’ with a ‘context of use’ (CoU). As a result, the way a CoU is managed changes throughout its lifecycle: the metadata plus its status now determines the action being performed. The CoU is either suspended or active. When active, you can define what is being replaced. There is no concept of ‘Append’, and the ‘Delete’ operation is replaced with the ability to suspend a CoU (which in the future can be made active again). The replace action also allows a many-to-one replacement, or a one-to-many replacement, allowing granular changes. Figure 2 summarizes the key differences between the two versions.
Figure 2. Critical Differences Between eCTD v. 3.2 and v. 4.0
Veeva is enhancing Vault RIM applications – Vault Submissions, Submission Publishing, Submission Archive, and Registrations – to support eCTD 4.0. Released data model changes are enabling sponsors to start enrichment projects, and support for Japan and the US is on the way. As other regions solidify their plans, additional local support will follow.
Read about Vault RIM support capabilities and Veeva’s Health Authority Gateway to learn more about how Veeva facilitates e-submission.