Preparing Monitors for Tomorrow’s Clinical Trials
In the first blog in our series, we discussed how the global pandemic facilitated the need for the industry to change the way clinical trials are run. In this blog, we’ll explore the role of the CRA in a post-COVID era.
Monitoring Challenges
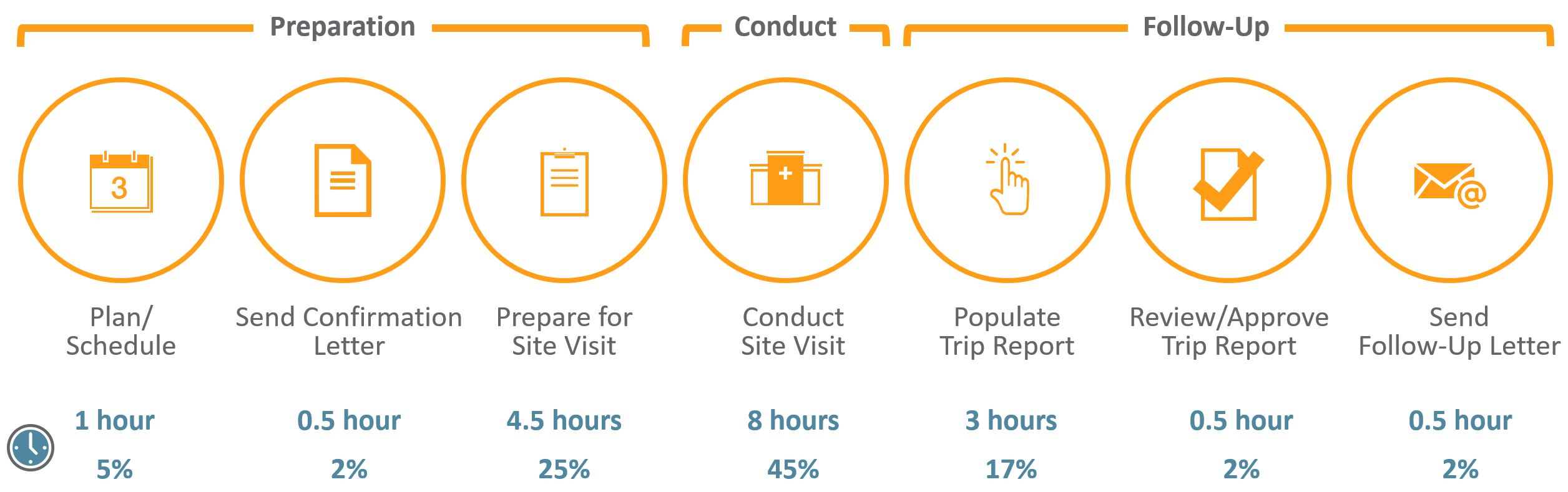
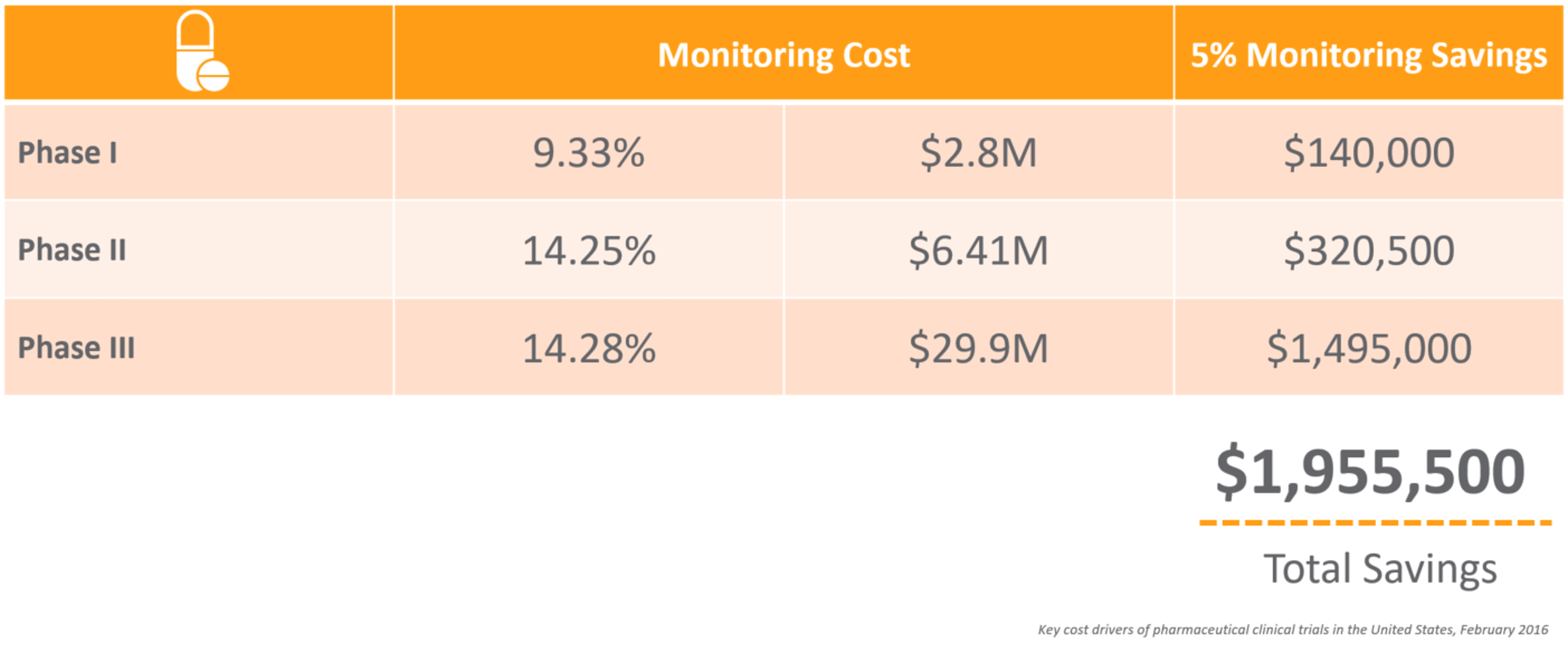
CRAs have a difficult job – from the extensive travel to the tedious time spent preparing for onsite visits and completing trip reports. For an average phase III trial for large pharma, monitors can spend up to 18 hours on just this task alone. With many top 20 pharma averaging over 40,000 monitoring trip reports per year,1 it’s not surprising that 25%-30% of total clinical trial costs are attributed to site monitoring.2
The time spent on monitoring visits is compounded by the reality that many CRAs work across 10+ systems, which are often siloed, adding effort and complexity to an already tough job.
Monitoring is an area ripe for optimization. Suppose you could reduce the time, effort, and cost of monitoring by 5%. What impact would that have on your clinical development costs and time to market?
Streamlining Clinical Trials: Adopting a Risk-Based Approach
One way to achieve significant time and cost savings, and increase study quality, is a sound RBM strategy that incorporates quality by design and centralized remote monitoring. Focusing on risks specific to a study’s critical data allows sponsors and CROs to be more productive and allocate resources more effectively. This risk-based approach also conforms with ICH GCP guidelines, enabling faster processes and workflows, and improving study quality.
By identifying critical data, performing risk assessments, and providing a more targeted SDV and remote monitoring strategy, clinical operations leaders can better mitigate risks and optimize trial outcomes.
The Shift in the Monitor’s Role
COVID has demonstrated just how much work CRAs can do offsite, such as reviewing regulatory documents and statistical reports. Armed with highly focused reports if they must go onsite, monitors can dedicate their time to value-add activities – ensuring drug storage procedures align with the protocol, supporting sites by creating an enrollment strategy, fixing known issues, and building stronger relationships with site personnel.
If there is a silver lining to this unprecedented global event, it’s that the industry has proven we can execute remote trials without 100% SDV, while upholding the same stringent efficacy, data quality, and data integrity standards. This approach has resulted in the first COVID vaccines being developed and delivered at record speeds. It will be interesting to see if the learnings we gained during this unprecedented time carry forward into normal monitoring processes post-COVID.
Check out the next blog in our series, where we dive into how to garner internal support for an RBM initiative and steps you can take to ensure a successful deployment and adoption.
1 De-identified average from Veeva customers.
2 Branch, E. (2016, April 30). Ways to Lower Costs of Clinical Trials and How CROs Help. Retrieved January 21, 2019, from https://www.americanpharmaceuticalreview.com/Featured-Articles/185929-Ways-to-Lower-Costs-of-Clinical-Trials-and-How-CROs-Help/