Survey Says! ClinOps Leading the Way
Veeva presents 2015 Paperless TMF Survey results at DIA
The use of paper in clinical operations and drug safety decreased significantly, as did the exchange of paper documents between CROs and sponsors, according to findings from the 2015 Veeva Paperless TMF Survey. These are positive indicators of an industry moving away from paper and embracing more mature processes.
Kathryn King, Vice President, Vault Clinical, presented results from the survey this week at the DIA Annual Meeting in Washington D.C. As always, the seminal industry event for collaboration and knowledge sharing.
 Sponsor-CRO collaboration and the large number of documents managed by clinical operations departments (typically more than half of all TMF documents) are obvious pain points for the industry to start migrating into electronic processes. It was not surprising then to see this drop in paper in clinops and a jump in the use of eTMF applications among sponsors and CROs. Likewise, the fact that almost 60% of respondents electronically archive documents is in line with this industry transformation.
Sponsor-CRO collaboration and the large number of documents managed by clinical operations departments (typically more than half of all TMF documents) are obvious pain points for the industry to start migrating into electronic processes. It was not surprising then to see this drop in paper in clinops and a jump in the use of eTMF applications among sponsors and CROs. Likewise, the fact that almost 60% of respondents electronically archive documents is in line with this industry transformation.
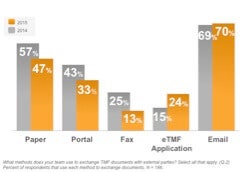
While these are encouraging signs, manual and paper processes remain. Email is far and away the most common method for exchanging documents between sponsors and CROs. Email offers few advantages over paper (It’s unstructured; lacks context; fraught with version control issues; etc.). Less than one third of respondents are leveraging esignature, or ecollaboration, or electronic document creation.
 Another important finding is respondents extensively using TMF data see significantly greater benefits, particularly improved inspection readiness and cost savings. This should be a clarion call for the industry to evaluate performance metrics as part of any ROI analysis when selecting eTMF systems.
Another important finding is respondents extensively using TMF data see significantly greater benefits, particularly improved inspection readiness and cost savings. This should be a clarion call for the industry to evaluate performance metrics as part of any ROI analysis when selecting eTMF systems.
The 2015 Veeva Paperless TMF Survey is a rich source of data on the maturity of TMF technologies, processes, and metrics. The information in this blog just scratches the surface and I encourage you to download the full report. This type of information is fundamental to learn how the industry can increase efficiency, compliance, and ultimately brings products to market faster.
Kevin McNulty is director, product marketing, Vault eTMF at Veeva