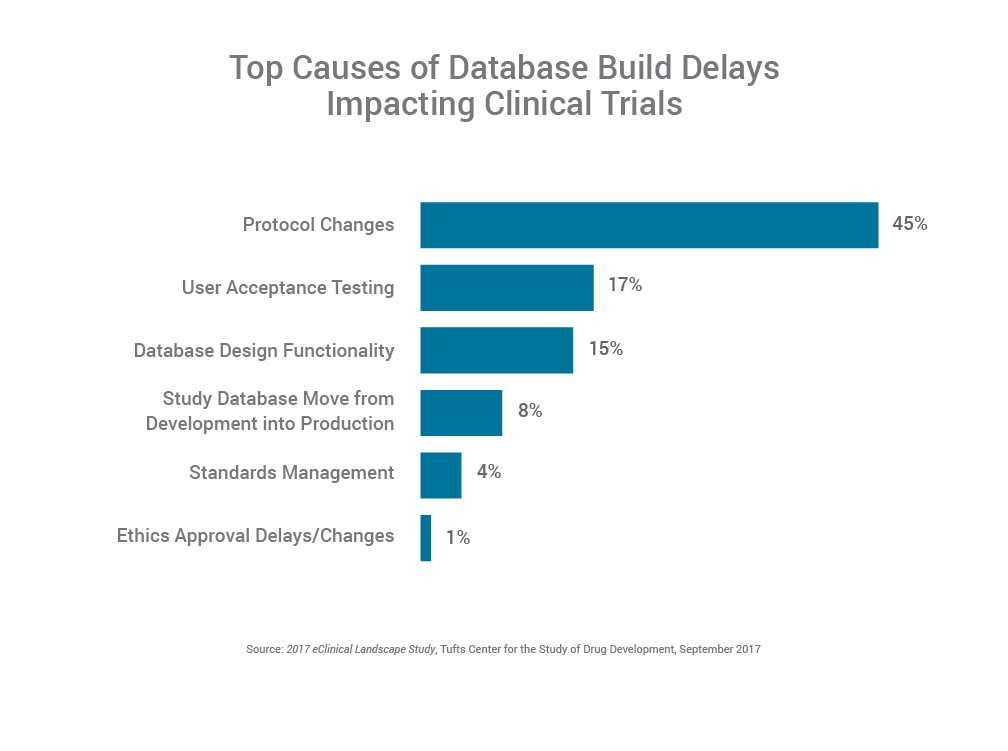
According to the Tufts eClinical Landscape Study, 45% of respondents say the most common cause for database build delays are protocol changes, underscoring the challenge data management professionals have in dealing with changes as they finalize the clinical trial database for the start of the trial. This highlights the need for standards and systems that support more flexible design and rapid development.
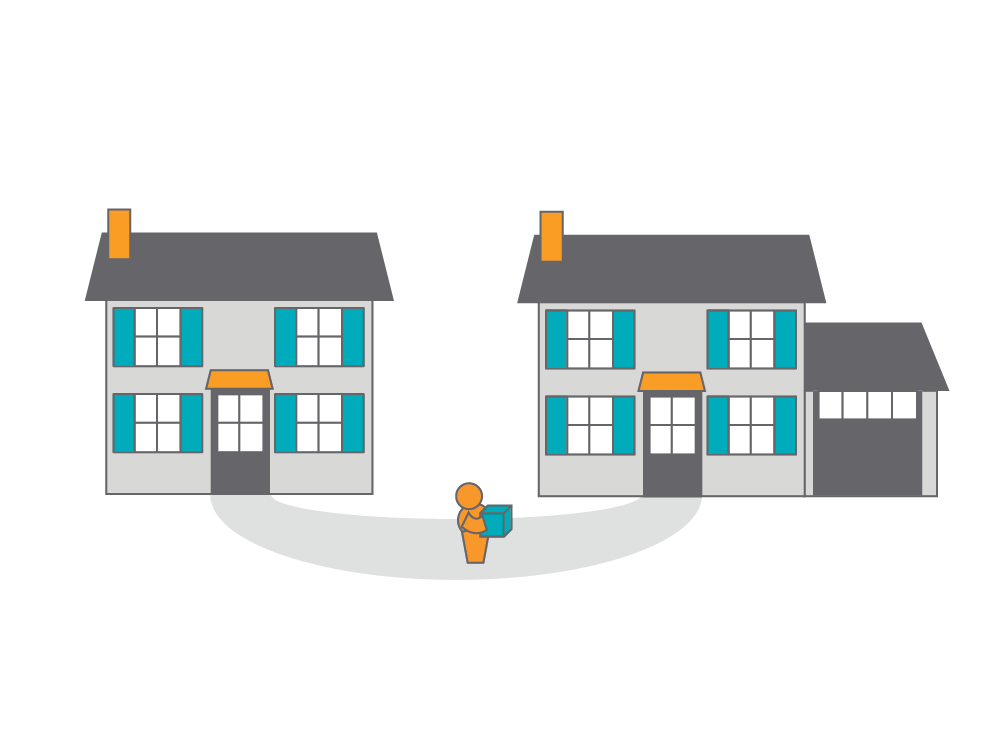
Building a house and building a study database both require careful planning and adherence to specifications. But when updates are needed, the processes couldn't be more different. Updating a protocol requires copying the database, making the changes, and migrating all the study data over to the new database. Veeva is rethinking the amendment process to update only the net changes, saving you time and money.

Introducing protocol amendments can incur extra costs and cause significant delays in your database build. Companies like Bioforum can execute mid-study changes with just a simple click of a button using Vault CDMS. Hear how their data managers make amendments effortlessly, maximizing productivity and saving them valuable time.
With the rise of personalized medicine and adaptive trials, amendments will only become more challenging. Veeva allows for an underlying database design that is as dynamic as the clinical trial itself. With an architecture designed to support multiple CRF designs and make real-time changes, Veeva delivers a better EDC that eliminates the need for migrations altogether.