Blog
Clinical Trials Can Be as Simple as Collect, Decide, and Act
Apr 26, 2017 | Richard Young
Apr 26, 2017 | Richard Young
Get drugs to market faster with transformed data collection
Clinical trials come in many shapes and sizes today, from the simple ‘feed and bleed’ to the complex, adaptive, multi-phase ‘umbrella’ approach, and every imaginable permutation in between. Defining what a clinical trial is in a few simple words is exceptionally difficult. It is however very easy to define the stages that are common in a clinical trial. First, we design our study, determining the data we wish to collect. Second, we use that accumulating data to decide how our clinical trial is progressing. Finally, we act upon those decisions.
Data is the foundation of these stages and the ability to manage it will determine our success in executing clinical trials. EDC systems are at the heart of data management and as a result, the clinical trial strategy.
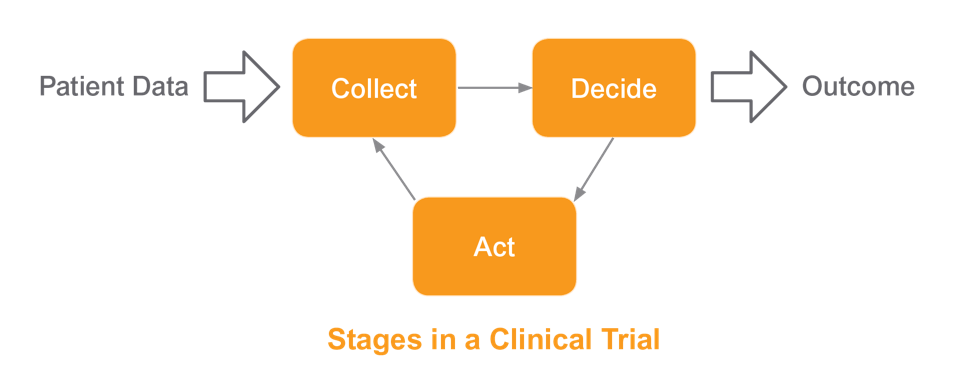 Collection. The EDC system needs to be able to handle all of the data generated in a trial and prioritize management of that data, based on relative value. Traditional EDC systems only manage case report forms (CRF) data. Today, CRF (also referred to as eCRF) data frequently represent less than 20% of overall study data. The remaining data is managed externally and leveraged manually. As a result, data is fragmented and sponsors in a trial are unable to review and manage all of their data. A modern EDC solution should deliver complete and concurrent data – all of the data, all of the time.
Collection. The EDC system needs to be able to handle all of the data generated in a trial and prioritize management of that data, based on relative value. Traditional EDC systems only manage case report forms (CRF) data. Today, CRF (also referred to as eCRF) data frequently represent less than 20% of overall study data. The remaining data is managed externally and leveraged manually. As a result, data is fragmented and sponsors in a trial are unable to review and manage all of their data. A modern EDC solution should deliver complete and concurrent data – all of the data, all of the time.
Decision Making. An extension of solving the data collection challenge is making decisions with confidence. The EDC system needs to transform the massive quantities of raw data collected into actionable insights. Enabling the reviewer to understand how the patient and study are progressing is critical to any successful strategy. When data is incomplete, it’s not possible to make confident decisions in a timely manner. Most EDC systems on the market today are not equipped to provide a complete and concurrent view of the patient at all times. They are unable to aid in real-time and high quality decisions based on a holistic view of the patient.
Action. As data is reviewed and decisions are made, the EDC system next needs to execute those decisions. Implementing changes to a trial requires best practices and rapid optimization of trial execution baked into the EDC system. Traditional EDC solutions may require days and even weeks of modification (including study migrations) before a decision can be fully implemented. Not only do these delays affect the implementation of critical decisions, but also slow the identification of the next key decisions.
Comprehensive data collection in clinical trials results in confident and quick decision making and real-time action. Visit our resource centre to learn how to transform clinical trials with better data collection.