Blog
How To Determine TMF Operating Model for Outsourcing
Aug 15, 2022 | Chris McSpiritt
Aug 15, 2022 | Chris McSpiritt
This is part one in a series about improving sponsor-CRO collaboration in TMF.
As outsourcing models flux, and best practices around TMF management evolve, sponsors and CROs must make key decisions around how they will work together. Over the last 10 years, sponsors have increasingly brought eTMF technology in-house to improve access, visibility, and control — but it has become clear there is no one-size-fits-all model.
Instead, it is critical to make the strategic decision that best supports your goals and establish expectations with all study partners up front. It can be a broad decision for the company or a more granular decision on a study-by-study basis, or other factors like therapeutic area.
This blog series will share what we’re seeing in the industry and provide guidance about how to determine which model is right for your organization.
Different Operating Models for TMF
There are a variety of operating models for TMF management. We’ll examine three models here:- CRO technology, fully or partially outsourced process and resources: The sponsor outsources the eTMF system, as well as process and resources, to a CRO. This might include a separate sponsor eTMF for sponsor documents. At the end of the study, the CRO migrates the TMF to the sponsor.
- Sponsor technology, fully or partially outsourced process and resources: The sponsor owns the eTMF system and the CRO(s) work in that system alongside the sponsor. The sponsor outsources some or all of the process and resources.
- Sponsor technology, insourced process and resources: The sponsor does not outsource either the eTMF or its management.
In any model, the sponsor should evaluate CRO capabilities for alignment — including their technology stack, their processes for providing access and enabling oversight, and their migration plans — and be clear with study partners up front which model they plan to use.
Benefits and Challenges of Each Model
Each operating model offers benefits and potential challenges for both sponsors and CROs. Sponsors should carefully consider those when assessing which model(s) to implement.
1. CRO Technology and Outsourced Process and Resources
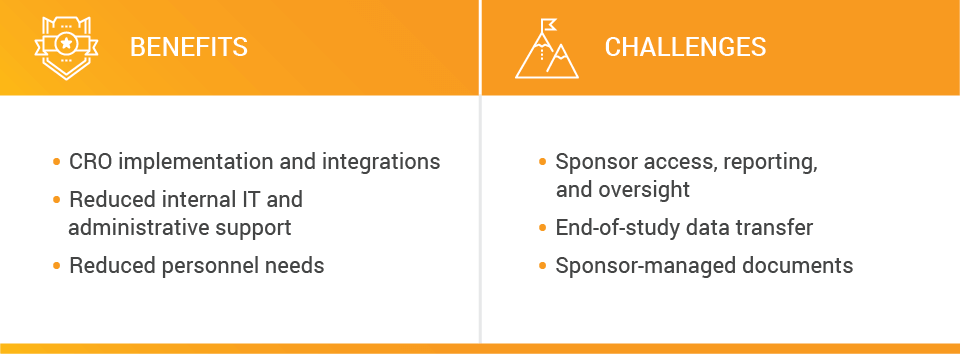
In this model, CROs maintain the efficiencies they’ve built in their own operations, including integrations with EDC, CTMS, and other applications, like billing systems. Sponsors might need less IT and administrative support internally, as CROs manage tasks like validation and updates. Sponsors might not need their own SOPs either.
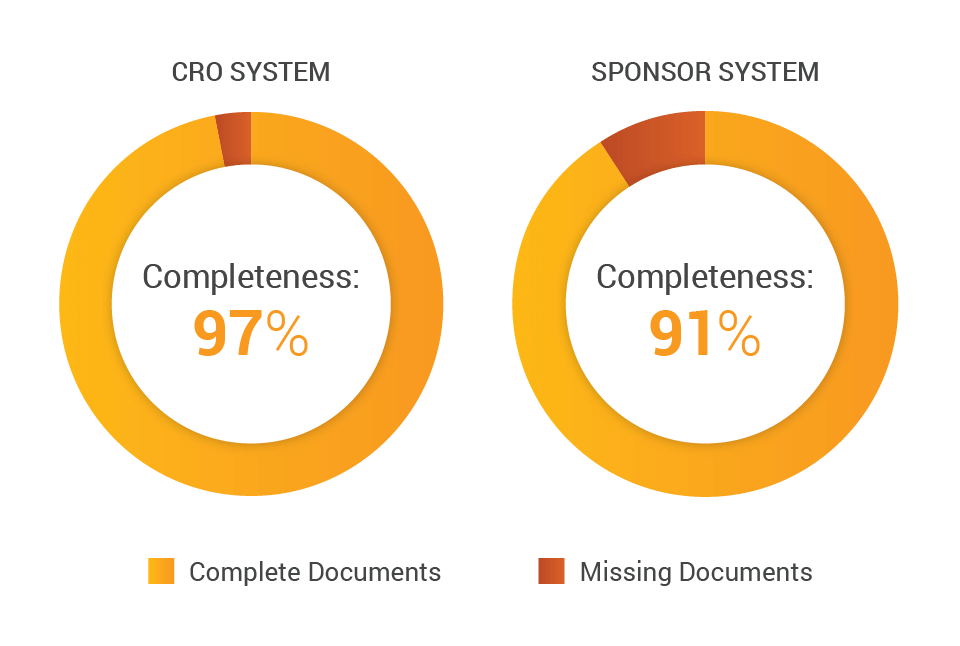
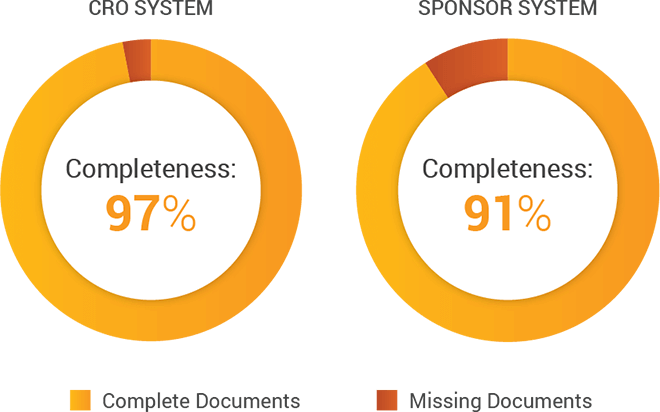
For one mid-size CRO we talked to, maintaining their own eTMF system ensures improved efficiencies and TMF completeness. According to the CRO’s chief information officer, “Separating our CTMS from our eTMF solution downgrades the effectiveness of trial management and documentation services. People are trained and empowered with an integrated set of processes that define how to work with the technology.”
Across their studies, this CRO found an aggregate 97% completeness metric when working in their own system versus 91% when working in the sponsor’s, driven by their team working outside the processes and technology on which they were trained.
However, potential challenges include sponsor reporting and oversight, including the ability to document issues. Depending on the level of access CROs grant sponsors, sponsors might not be able to perform real-time oversight of their TMF due to inability to see all documents.
Data transfer is another potential challenge, including migration at the end of the study. Sponsors will also need to consider where they host oversight documents and whether they maintain a separate system to do that.
| BENEFITS | CHALLENGES |
|---|---|
|
|
2. Sponsor Technology and Fully or Partially Outsourced Process and Resources
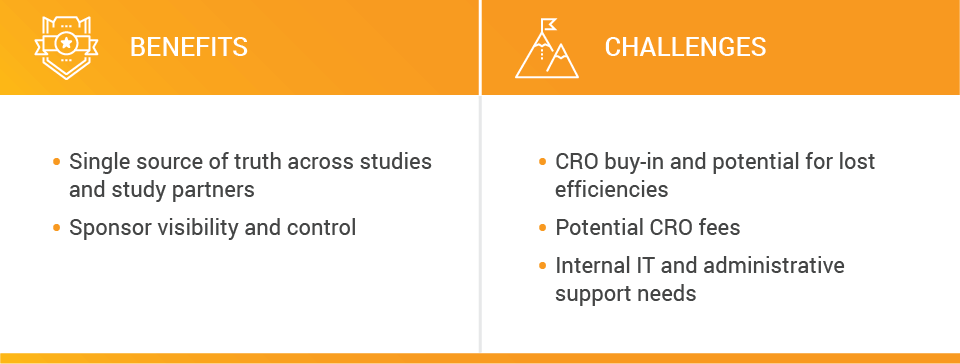
In this model, sponsors maintain a single source of truth across studies and study partners — their own eTMF. Potential benefits include increased oversight, visibility, and control. This model also enables sponsors that work with a large number of CROs to standardize their processes.
One mid-sized biopharmaceutical company moved their eTMF in-house after conducting a TMF rescue from a CRO. The CRO didn’t provide the visibility into TMF health that they required, and they confirmed quality problems with an on-site audit. Since then, they’ve developed master plans at the program and study levels, consolidated training across vendors, and shared metrics to improve collaboration with their CRO partners.
“This program not only drives TMF quality but it also positively impacts how we interact with our CROs,” the biopharmaceutical’s TMF owner said. “Our CRO relationship is an ongoing engagement, and it does require work as it evolves.”
For another top 20 pharmaceutical company, the model fit with their growth plan: “This model made sense with our complex outsourcing model, focus on sponsor oversight, and need for more transparency and accountability in our growing organization with a global footprint.”
As regulators continue to focus on the TMF, it’s critical that sponsors can demonstrate oversight. In the MHRA’s most recent GCP Inspections Metrics Report, the largest category of major findings was record keeping/essential documents, including findings noting a lack of effective oversight QC and no review of the audit trail by sponsors, which this model can help address.
However, potential challenges include securing CRO buy-in and ensuring continued efficiencies, as well as potentially increased CRO fees.
| BENEFITS | CHALLENGES |
|---|---|
|
|
3. Sponsor Technology with Insourced Process and Resources
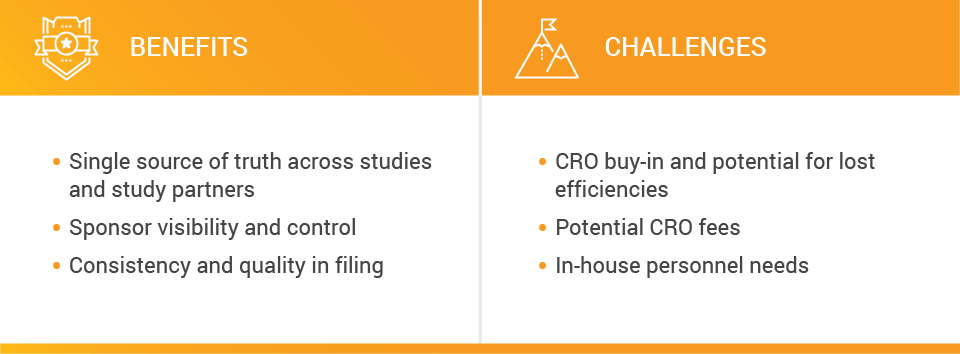
In this model, sponsors likely have a centralized document processing team. We tend to see this model in larger or enterprise organizations where more study management activities are performed in-house, as well as among sponsors that outsource site management. In the latter model, the CRO collects content and the sponsor reviews and processes it. Potential benefits for this model align to the benefits of the previous model, including a single source of truth across studies and study partners.
For one top 20 pharmaceutical company, the model enabled them to more effectively respond to work-from-home mandates and streamline operations across countries.
“We created a centralized model between two vendors in three regions to serve as indexers and to provide quality controls,” said the company’s head of TMF. “This improved our business continuity, and we saw an increase in quality and consistency in filing.”
Potential challenges include heavy investments from the sponsor in internal personnel, as well as lost efficiencies and expertise that a CRO can bring to bear.
| BENEFITS | CHALLENGES |
|---|---|
|
|
The majority of sponsors outsource at least some part of their TMF operations, so you can use the following steps to align with study partners and improve collaboration and inspection readiness.
Picking the Right Model for Your Organization
- Decide what your organizational priorities and characteristics are. This will help you select the right operating model(s) for your organization. These considerations might include:
- Efficiency
- Oversight and visibility
- Control
- Flexibility
- Clinical trial volume
- Number of study partners
- Evaluate potential CROs to determine whether they invest in technology and process with a focus on TMF quality. Ensure the correct capabilities exist to support your priorities.
- Choose your model and align with all study partners. No matter which model you choose, make sure you’re aligned from the beginning. If CROs work in their own systems, define expectations for visibility and oversight, as well as bridging SOPs if needed to close the gap between sponsor and CRO SOPs. CROs are also familiar with the concept of working in a sponsor’s system, but it’s important to define that requirement if you choose that model. Then, you can jointly define a TMF Plan and set KPIs the CRO needs to meet for completion, quality, and timeliness.
Learn more ways to improve sponsor-CRO collaboration and explore other TMF innovations by accessing content from the inaugural TMF Innovation Forum. Watch sessions on-demand now.