Veeva Vault CTMS
Know What’s Going On In Your Trial and Take Action Sooner
Manage trials end-to-end with a modern CTMS.
Announced 2016 Status Very Mature Customers 100+
Find out why every biopharma needs a CTMS
Overview
Easily manage insourced
and outsourced studies
with Vault CTMS
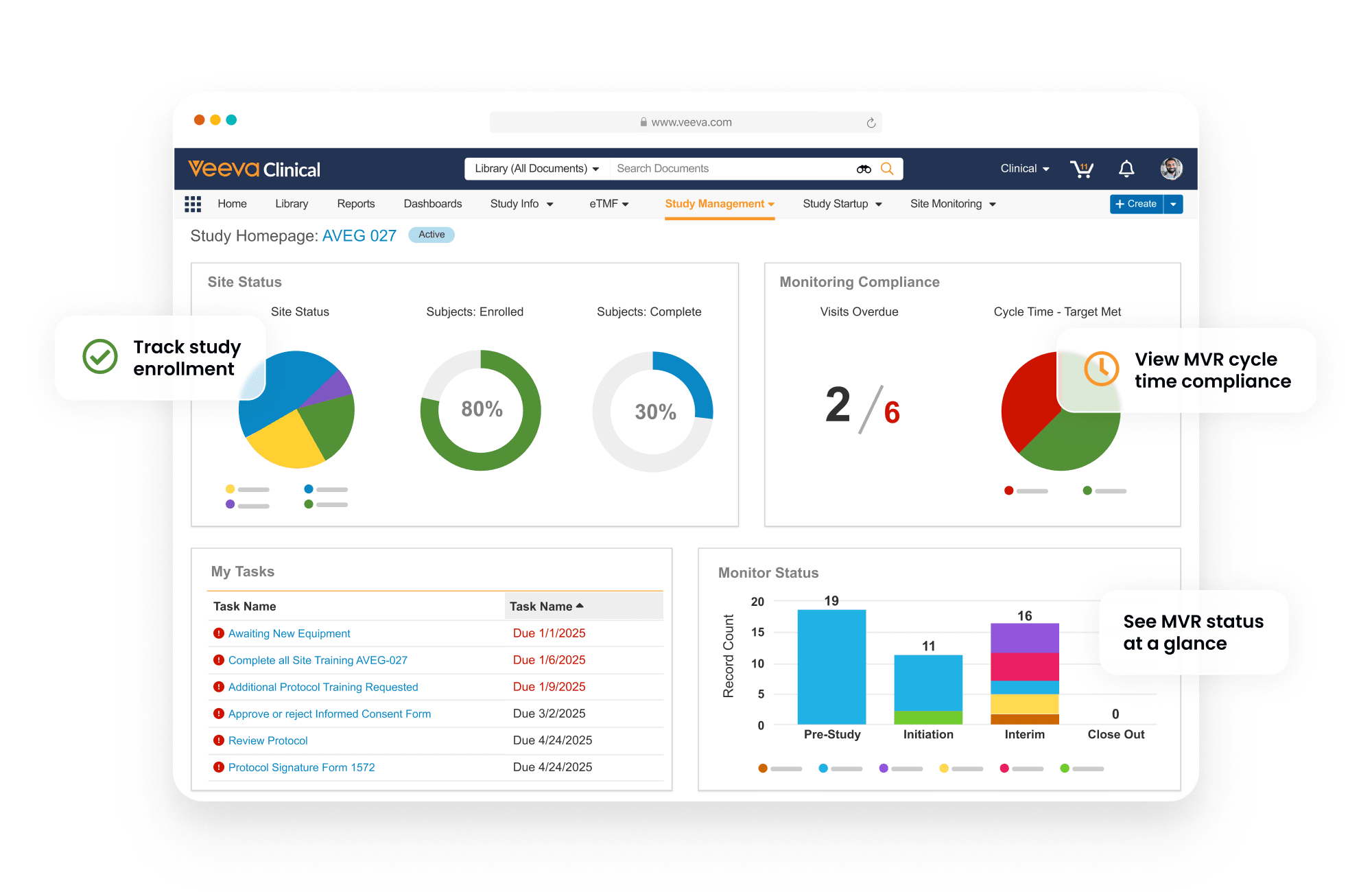
Enterprise trial management system
Veeva Vault CTMS provides end-to-end study management and monitoring capabilities for insourced and outsourced trials.
Connected with Vault EDC
Vault CTMS is connected with Vault EDC to support enrollment, monitoring, payments, and navigation to casebooks directly from within Vault CTMS.
Full-featured to support trial needs
Dashboards and reports track key indicators, including enrollment and milestones, with drill-down to take action. Monitoring visit reports support automation and dynamic question branching. Trip reports are automatically filed within Vault eTMF. Issues and Protocol Deviations are logged as needed and routed through resolution workflows to ensure closure. CTMS Transfer automates the daily transfer of data between sponsors and CROs using Vault CTMS.
Impact
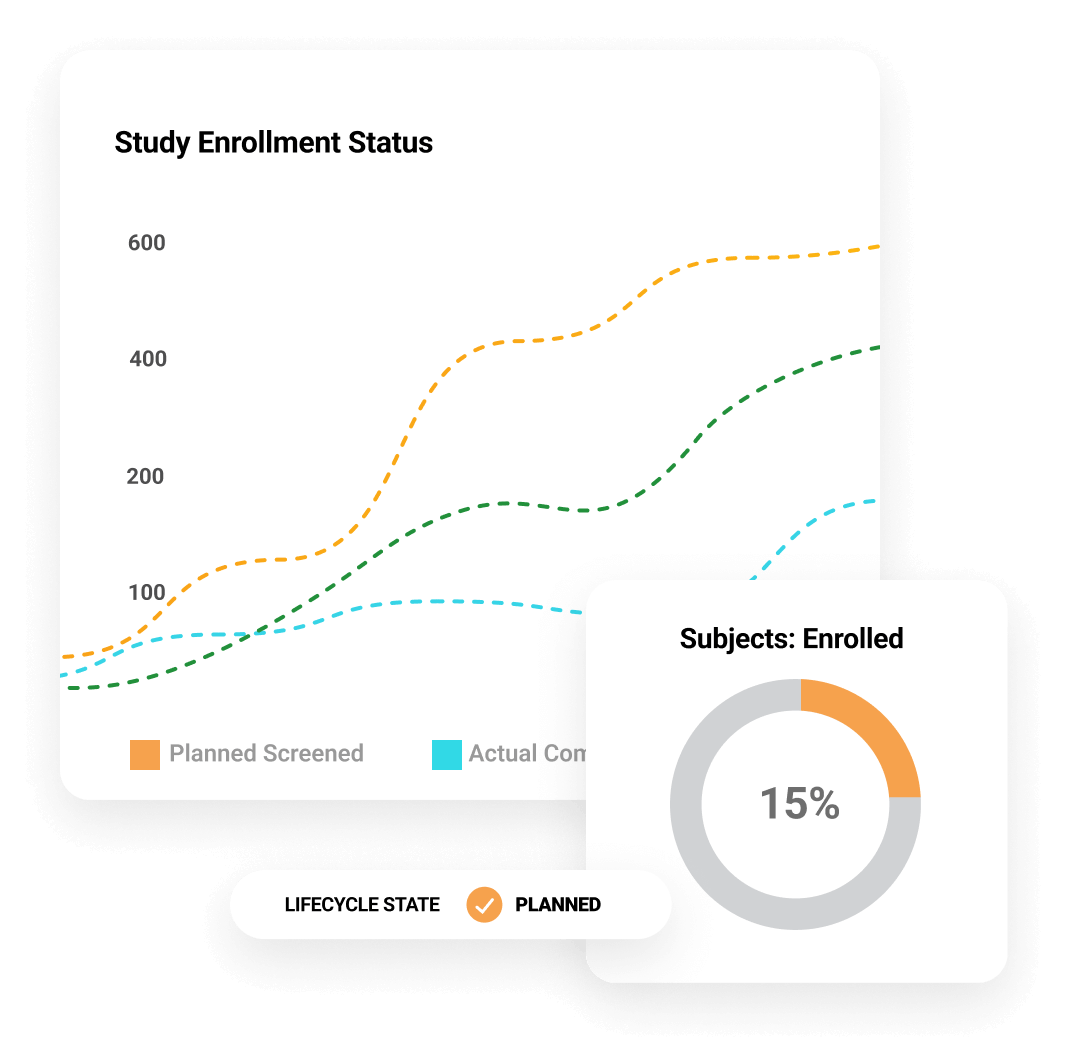
Centralize data and documents in one flexible system
30%
reduction in monitoring costs
50%
less time to author visit reports
80%
faster identification of issues
Why Vault CTMS
Faster, higher quality trials
Customer Success
Streamlining trial management
for 200+ sponsors and CROs










Resources
Explore and Learn
Read eBook
Discover why a CTMS is essential for every biopharma

Watch customer video
See how GSK simplifies site monitoring

Watch customer video
See why eTMF and CTMS are better together for Jazz

Read case study
Top 20 pharma: CTMS transformation best practices

Watch demo
Effectively plan and conduct monitoring visits
Learn more
Achieving effective study oversight with a modern CTMS

Read white paper
Modernizing the hub of clinical operations

Watch video
See how Vault CTMS streamlines trial management
Hear from a top 20 pharma
Making the case to replace legacy CTMS
