Blog
Simplify CRAs’ Return to Sites with Offline Monitoring
Apr 14, 2021 | Henry Galio
Apr 14, 2021 | Henry Galio
As more CRAs prepare to return onsite for monitoring visits, optimizing the trip report process should be a priority for sponsors and CROs to drive operational efficiencies and make things easier for monitors.
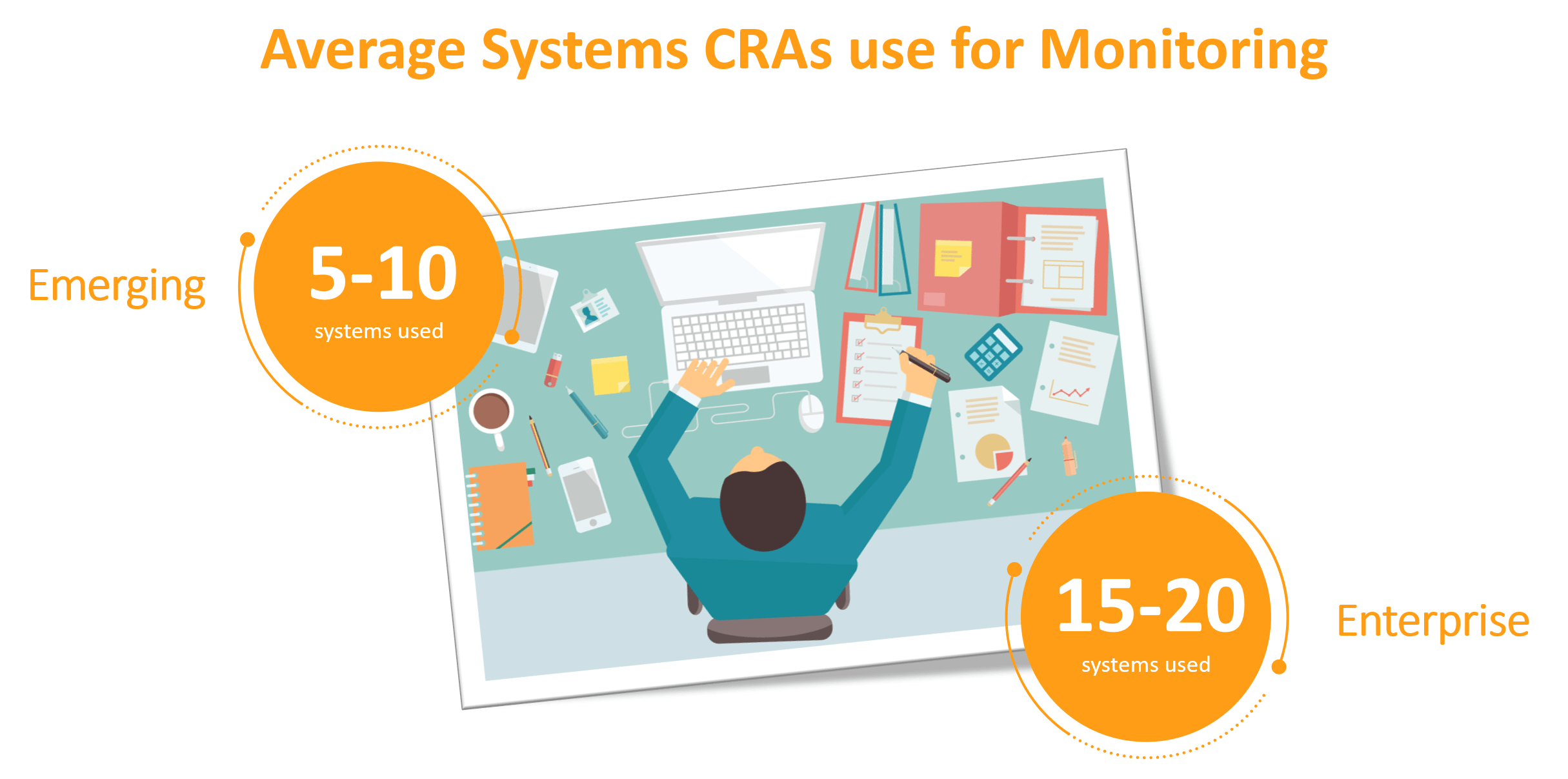
Over the last 20 years working in CTMS software and process optimization, I have conducted multiple time and motion studies with emerging and enterprise pharma, biotech, and medical device companies, as well as CROs. These studies help companies understand all of the systems CRAs use to prepare, conduct, and finalize monitoring visits. The findings uncover an urgent need for improvements.
There are three main time and efficiency blockers:
- CRAs rely on trackers (Excel, Word, and paper) to transpose information from reports, notes, and other sources, then re-enter into CTMS.
- Too many reports, systems, and steps are required to conduct monitoring.
- CRAs are unable to use CTMS when onsite for monitoring due to connectivity issues and limitations.
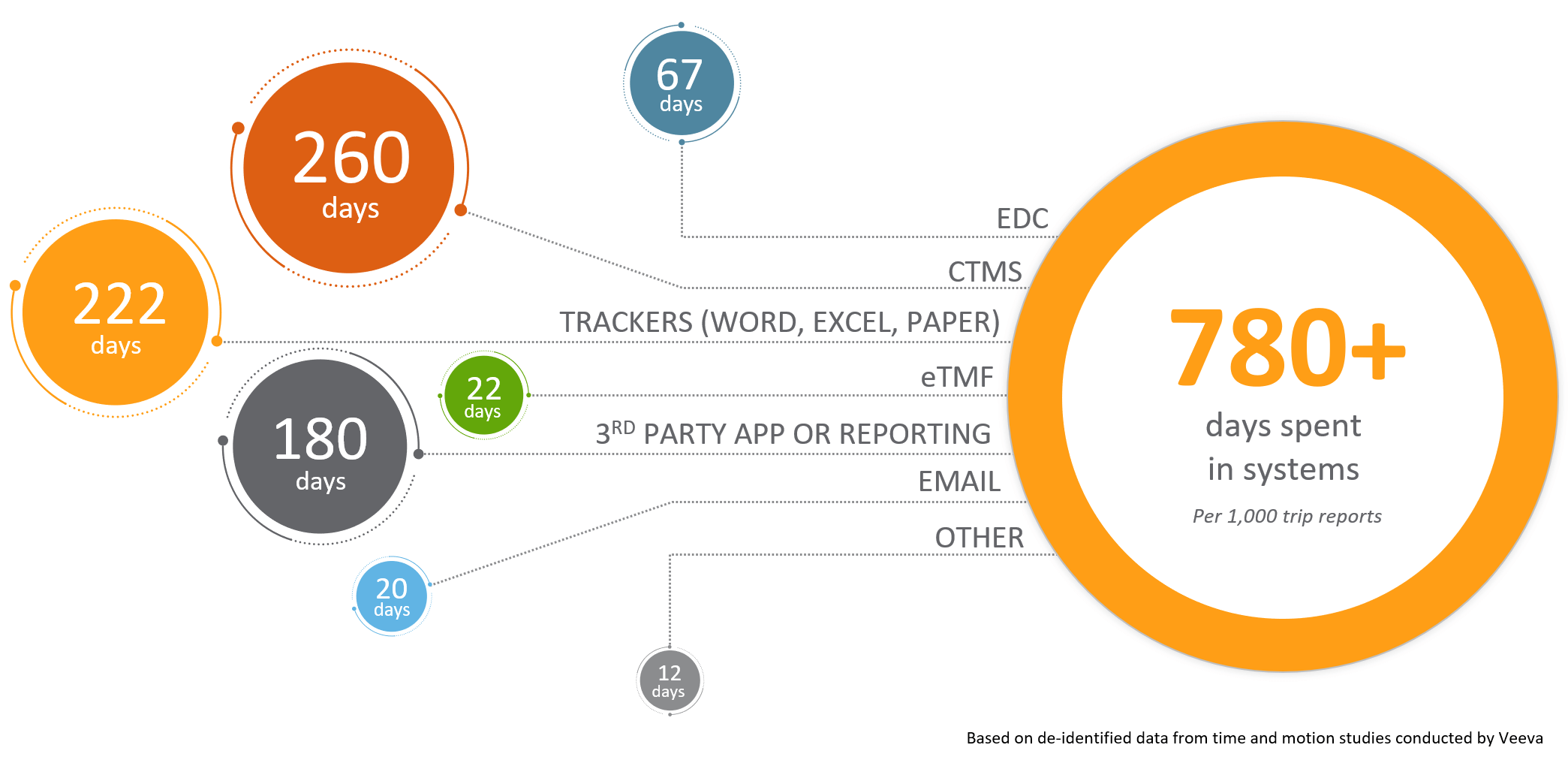
Because of these issues, a lot of time is spent monitoring. For every 1,000 trip reports, CRAs spend more than 780 days working in various systems. CRAs noted that VPN connection challenges and non-intuitive interfaces in legacy CTMS technology make their work more difficult. Many monitors supplement with Word, Excel, or paper trackers because of these usability challenges, resulting in double data entry.
Not only is this process inefficient, but it makes things more difficult for monitors who already have a tough job. If you could simplify monitoring by allowing CRAs to do direct data entry without internet connection, continue to work while traveling, and ensure better data quality with on-the-spot capture, would you do it?
If the answer is yes, watch this demo to learn how to make monitoring easy for CRAs and maximize efficiency with Vault Clinical Suite.