Top Takeaways from TMF Summit 2019
eTMF technology has evolved since its inception around a decade ago. The attendees of the 8th TMF Summit in Orlando would agree as they explored the modern tools and techniques available today to transform trial execution.
The following top insights from the conference will help evolve your TMF processes.
1. Better metrics yield better results
Metrics and dashboards are most effective when they provide real-time visibility into essential key performance indicators (KPIs) and actionable insights. For example, an incomplete TMF report along with the visibility into the list of missing documents and where to find them.
In his presentation on effective collaboration, Mark Romano of Veeva Systems advised on using metrics as early as the study planning stage for full visibility into the end-to-end trial process. He provided examples of both historic and forward-looking metrics for making TMF processes more efficient (See figure below).
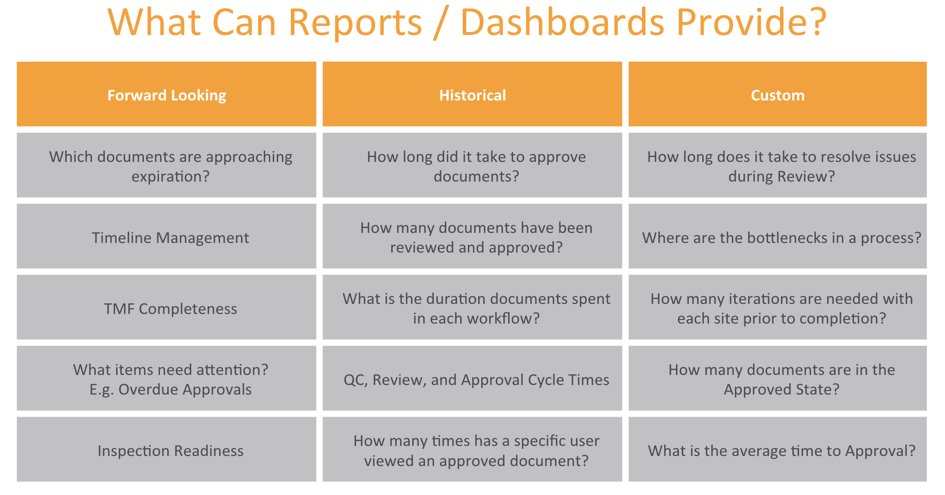
2. Inspection readiness requires TMF collaboration
A challenge in maintaining an inspection-ready TMF is the seamless transfer of TMF content from external sources such as regulatory, safety, manufacturing, and legal systems. Mark shared how a unified approach streamlines all processes in a clinical trial delivering full transparency and simplifying collaboration.
“Seamless data exchange between the eTMF and other clinical applications such as CTMS and study start-up optimizes active TMF management,” said Mark. “A unified environment enhances visibility, oversight, and collaboration enabling a constant state of inspection readiness.” Learn more by viewing the full presentation below –
3. Effective trial management needs good governance
Having modern clinical applications combined with well-aligned business processes is essential for effective trial management. Presenters from Lilly, Pfizer, and others underscored the importance of regular training, system maintenance, and business process automation for active TMF management.
A training program must support the needs of new users as well as existing users. Victoria Ho, Clinical Operations Director, Gilead, urged the audience to take the time to learn the technology and ensure internal as well as external stakeholders have strong knowledge of the TMF Reference Model.
Regular maintenance and upgrades of all applications – cloud or on-premises – are crucial to keeping up with the latest industry best practices and optimizing efficiencies. While cloud application upgrades are easier and seamless, business processes must be revisited for updates after each upgrade. For example, SOPs may need to be updated to reflect new workflows created after a system upgrade.
Lastly, good governance is a must for continuous process improvement. Eli Lilly’s Patricia Fulton, Quality Consultant, TMF, shared a well-defined governance structure, which included:
- Critical resources and their core responsibilities
- Maintenance of existing tools and systems
- Comprehensive training
Next steps
eTMF technology has evolved rapidly, but not all organizations have fully matured their TMF. Veeva’s TMF Maturity Model, a complimentary tool, helps organizations assess the current state of their TMF compared to industry benchmarks with a roadmap for improvement.
Take the assessment and receive tactical steps to improve inspection readiness and trial performance.