Veeva Summit 2018 Recap: Disrupting Quality Management
The major quality announcements for this year’s Veeva Summit were connecting Veeva Vault QMS with Veeva Vault RIM and extending the Veeva Vault Quality Suite with Veeva Vault Training.
Connecting applications will help streamline key quality processes and provide greater visibility to all stakeholders. Bristol-Myers Squibb (BMS) and Upsher-Smith Laboratories discussed how standardizing on Vault for quality and regulatory will support a seamless change control process across functional areas – increasing efficiency and enabling faster, more informed decisions.
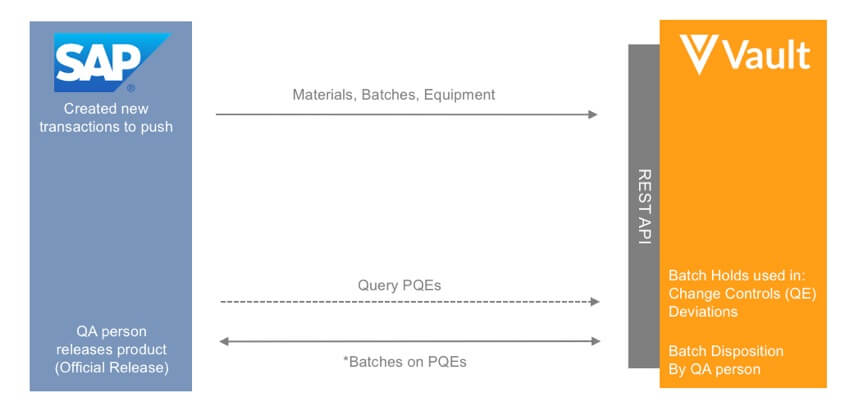
After going live with Veeva Vault QualityDocs in six weeks and Vault QMS in 12 weeks, Upsher-Smith is currently managing change control, CAPAs, deviations, complaints, and all controlled content on the Vault platform. The company also integrated Vault QMS with SAP to reduce the opportunity for errors in the batch release process. To learn more, see Upsher-Smith’s presentation here.
Challenges with global operations are driving many companies to re-evaluate their legacy solutions. In their presentation “Modernizing Quality Management,” BMS shared their strategy on simplifying and integrating quality systems across the lifecycle – reducing the number of systems from five to one and key processes from 21 to six. With “fewer steps, fewer handoffs, and fewer approvals,” processes will be faster and more efficient. BMS’ transition to the cloud will also support seamless processes with CMOs, CROs, and other partners – a key reason in their decision to modernize.
Gilead Sciences is using Vault to collaborate and manage data generated by partners for greater oversight in the development and manufacturing of their products. With the expansion of Vault to include enterprise-wide quality content, Gilead developed best practices on implementing and migrating to a cloud-based quality solution. Designing a flexible system and fully engaging with the business made it easier to incorporate acquired companies and are key factors in the overall success of the project.
Gaining a more holistic view of quality reduces risk and helps improve processes. With the introduction of Vault Training, companies can manage the qualification and compliance training status of their employees and better understand how training is tied to quality events and processes. With a unified quality suite, Foamix – a specialty pharmaceutical company – can quickly pull a training record or SOP, to demonstrate control and compliance in the event of a regulatory inspection.

Good documentation practices is also important for audits and inspections. Sysmex shared how they use Vault QualityDocs to track all changes made to a document, why those changes were made, and the last time the document was reviewed. By digitizing processes, quality teams ensure key information is captured and accessible to all relevant parties. Download Sysmex’s presentation.
A year ago, the Veeva quality community meeting created a forms working group to address the challenges many companies faced in managing forms. At Summit, the team presented their progress – proposed terminology on forms to reduce confusion as well as implementation best practices for forms as documents. The current guide on forms will be sent to the quality community for feedback and finalized by the end of the year, and will be expanded next year to include forms as objects.
With a record turnout of over 100 attendees, two new working groups have been proposed to start in 2019: audit and inspection readiness, and batch management including the lot disposition process. For more information on forms or to participate in the working groups, please email Bob Kenney.
At the Veeva Global Summit, companies learned how to disrupt and improve quality management by leveraging modern technology. Click here to watch the highlights from this year’s general session. We look forward to seeing you next year!